A Ratiometric Fluorescent Probe for Detecting Hydrogen Peroxide and Its Application
A fluorescent probe and hydrogen peroxide technology, applied in the field of analytical chemistry, to achieve the effects of easy preparation, convenient use, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
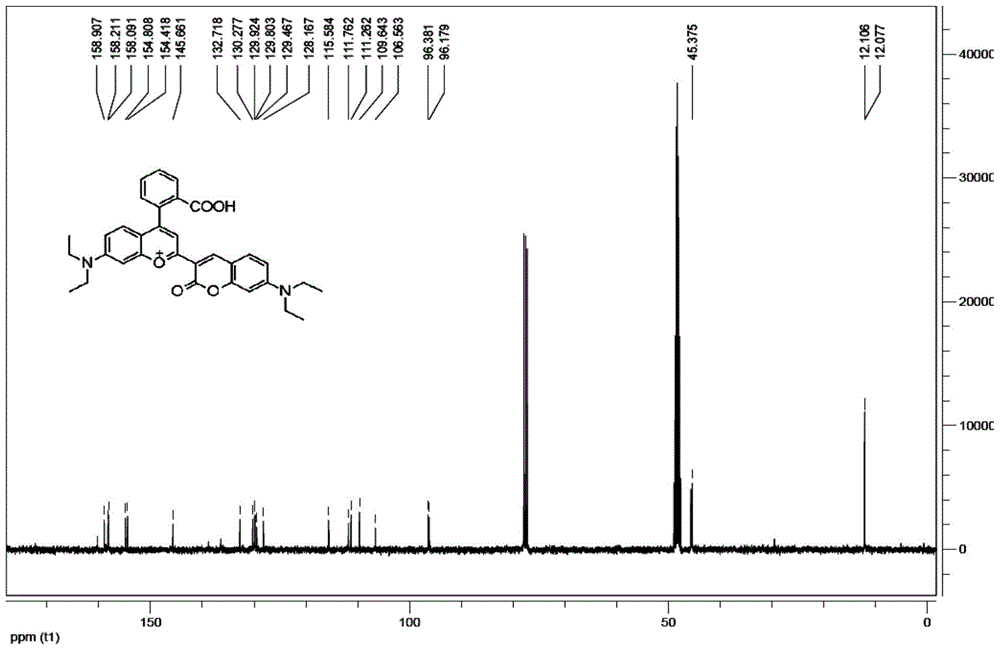
[0027] Example 1: Preparation of 2-(3-(7-diethylamino)coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyran perchlorate:
[0028] Dissolve m-diethylaminophenol (1.65g, 10mmol) and phthalic anhydride (1.48g, 10mmol) in 20ml of toluene, reflux for 6 hours under nitrogen protection, add 30ml of 36% NaOH aqueous solution after cooling at 60°C, Continue the reaction for 2 hours, then cool at room temperature, adjust the pH to 5 with 1N HCl, filter, dry, and recrystallize to obtain light gray intermediate 1, which is: 2-(4-diethylamino-o-hydroxyphenyl) benzoic acid.
[0029] Add intermediate 1 (1.56g, 5mmol) and 3-acetyl-7-ethylenediamino-coumarin (1.29g, 5mmol) to 6ml of concentrated sulfuric acid, react at 90°C for 12 hours, and cool to room temperature Finally, pour it into ice water, add 70% perchloric acid, filter, and purify the solid by column chromatography to obtain a dark blue product, which is: 2-(3-(7-diethylamino) Coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyra...
Embodiment 2
[0032] Example 2: 2-(3-(7-diethylamino) coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyran perchlorate to different substances selective identification
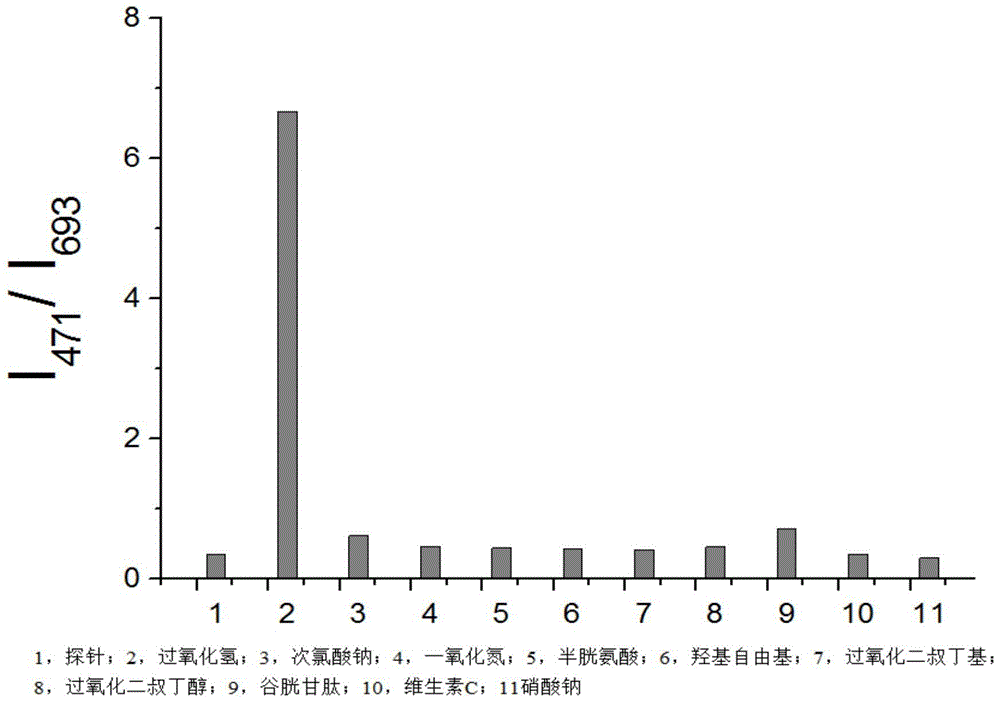
[0033] Prepare ten 5 mL portions of 5 mM 2-(3-(7-diethylamino)coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyran perchlorate in PBS solution (containing 5% methanol), and then sequentially added 50 μL of hydrogen peroxide, sodium hypochlorite, nitric oxide, cysteine, hydroxyl radicals, di-tert-butyl peroxide, Di-tert-butanol peroxide, glutathione, vitamin C and sodium nitrate in PBS. Fluorescence detection (λ Ex =380nm); Calculate the fluorescence intensity ratio I at 471nm and 693nm in each system 471 / I 693 , see the result image 3 . Wherein it is shown that the 2-(3-(7-diethylamino)coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyran perchlorate is effective against peroxidation Hydrogen selectivity is significantly enhanced.
Embodiment 3
[0034] Example 3: 2-(3-(7-diethylamino)coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyran perchlorate and hydrogen peroxide linear relationship
[0035] Prepare 1 mL of PBS solution system of hydrogen peroxide with a gradient of 0-5 mM concentration, and then add 9 mL of probe PBS solution (containing 5% methanol) with a concentration of 50 μM for fluorescence detection (λ Ex =380nm,λ Em =471nm and 693nm), calculate the fluorescence intensity in each system, and the fluorescence intensity ratio I471 / I693 at 471nm and 693nm, establish the standard curve of fluorescence intensity and hydrogen peroxide concentration, the standard curve is shown in Figure 4 .
[0036] Fluorescence probe 2-(3-(7-diethylamino)coumarin)-4-(2-benzoic acid)-7-diethylamino-benzopyran perchlorate of the present invention The lower limit of detection was 0.079 μM (S / N=3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com