S-triazine derivative, and preparation method and application thereof
A technology of s-triazine and its derivatives, which is applied in the direction of chemical instruments and methods, instruments, analytical materials, etc., and can solve the problems such as the decrease of fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
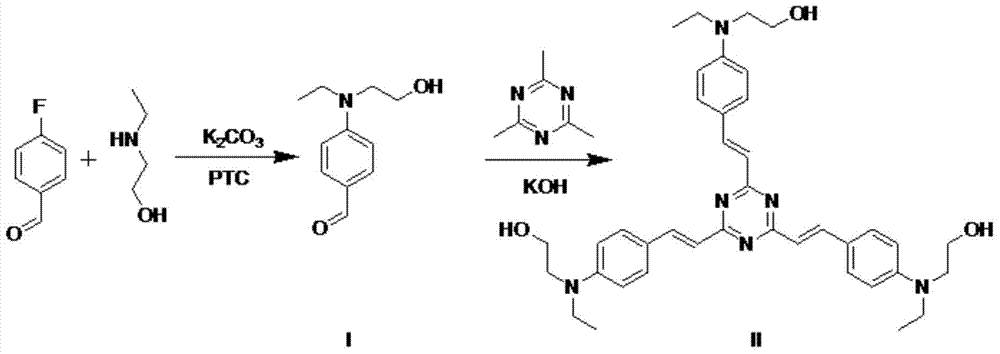
[0028] The preparation method of s-triazine derivative (compound II):
[0029] (1) Synthesis of 4-(N-ethyl-N-hydroxyethyl)aminobenzaldehyde (I)
[0030] In a round bottom flask, weigh 0.5 g p-fluorobenzaldehyde and 0.72 g 2-(ethylamino)ethanol, 0.005 g CTAB, 5 g K 2 CO 3 , dissolved in 50ml DMSO, and magnetically stirred in an oil bath at 95°C for 24h. The mixture was then poured into 100ml of distilled water, extracted twice with 50ml of dichloromethane, the organic layers were combined and dried over anhydrous magnesium sulfate, the solvent was removed with a rotary evaporator, and the resulting residue was purified by silica gel column chromatography (ethyl acetate Esters: Petroleum ether = 1:10) to obtain compound (I) in the form of yellow oil.
[0031] 1 H NMR (CDCl3, 400MHz): δ(ppm) 1.18(t, 3H, J=6.8Hz), 3.54(t, 2H, J=5.6Hz), 3.51-3.46(m, 2H, J=7.2Hz), 3.81(t,2H,J=5.6Hz),4.28(t,1H),6.71(d,2H,J=8.4Hz),7.62(d,2H,J=8.4Hz),9.60(s,1H). Calcd for C 11 h 15 NO 2 :C,68....
Embodiment 2
[0036] (1) Synthesis of 4-(N-ethyl-N-hydroxyethyl)aminobenzaldehyde (I)
[0037] In a round bottom flask, weigh 0.2g of p-fluorobenzaldehyde and 0.6g of 2-(ethylamino)ethanol, CTAB0.01g, K 2 CO 3 3g, dissolved in 30ml DMSO, stirred magnetically in an oil bath at 80°C for 30h. Then the mixture was poured into 90ml of distilled water, extracted three times with 60ml of dichloromethane, the organic layers were combined and dried over anhydrous magnesium sulfate, the solvent was removed with a rotary evaporator, and the resulting residue was purified by silica gel column chromatography (ethyl acetate : Petroleum ether=1:5) to obtain yellow oily compound (I).
[0038] (2) Synthesis of 2,4,6-tris[4-(N-ethyl-N-2-hydroxyethyl)styryl]-s-triazine (II)
[0039] In a round bottom flask, 0.1 g of 2,4,6-trimethyl-s-triazine and 0.1 g of KOH were added to 10 ml of ethanol. After stirring at 80° C. for 30 min, 5 ml of ethanol solution of Compound I with a concentration of 0.05 g / ml was sl...
Embodiment 3
[0041] (1) Synthesis of 4-(N-ethyl-N-hydroxyethyl)aminobenzaldehyde (I)
[0042] In a round bottom flask, weigh 1 g of p-fluorobenzaldehyde and 2 g of 2-(ethylamino)ethanol, CTAB 0.02 g, K 2 CO 3 10g, dissolved in 100ml DMSO, and magnetically stirred in an oil bath at 110°C for 30h. Then the mixture was poured into 100ml of distilled water, extracted twice with 100ml of dichloromethane, the organic layers were combined and dried over anhydrous magnesium sulfate, the solvent was removed with a rotary evaporator, and the resulting residue was purified by silica gel column chromatography (ethyl acetate Esters: Petroleum ether = 1:8) to obtain compound (I) in the form of yellow oil.
[0043] (2) Synthesis of 2,4,6-tris[4-(N-ethyl-N-2-hydroxyethyl)styryl]-s-triazine (II)
[0044] In a round bottom flask, 0.5 g of 2,4,6-trimethyl-s-triazine and 1.0 g of KOH were added to 40 ml of ethanol. After stirring at 110° C. for 30 minutes, slowly add 60 ml of an ethanol solution of Compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com