2-phenyl propionic ester derivative and preparation method and application thereof
The technology of a drug and an alkyl group, which is applied in the field of new intermediates and their preparation, can solve the problems of many side reactions, difficult to obtain, etc., and achieves the effects of simple preparation and difficult to obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
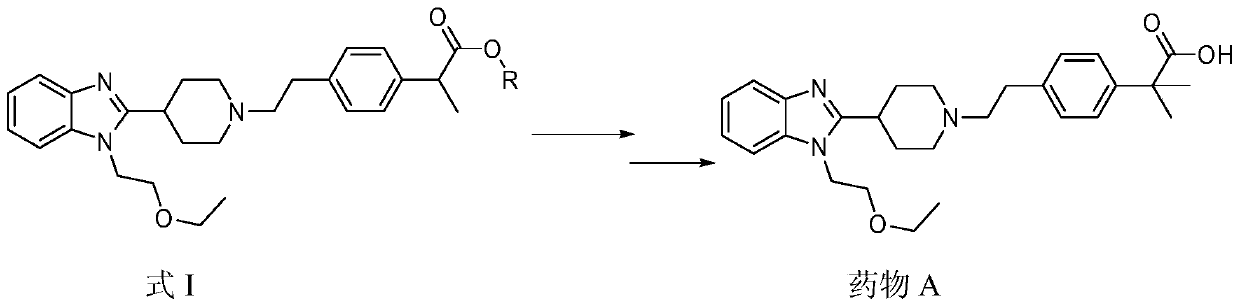
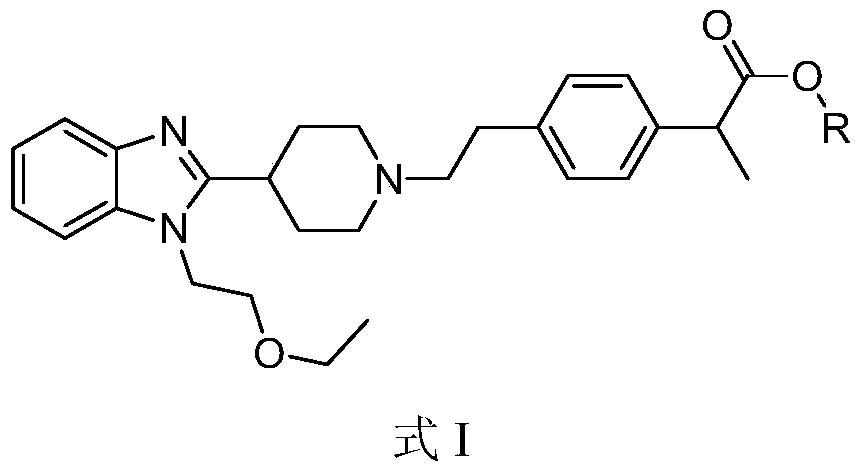
[0044] Example 1 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]phenyl]-propionic acid methyl Synthesis of esters
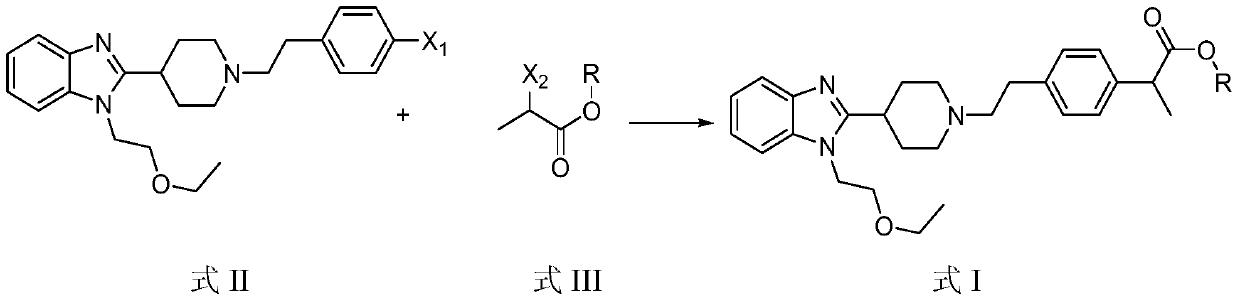
[0045] Add 8.24g of 4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]chlorobenzene to 200ml N,N-di Add 2.12g of bipyridine nickel bromide and 0.09g of manganese dioxide (activated) to methylformamide, add 4.18g of methyl 2-bromopropionate in N,N-dimethyl Base formamide solution, after the dropwise addition was completed, the reaction was incubated at 100°C, and the reaction was stopped after the disappearance of one of the raw materials was detected by HPLC.
[0046] The reaction solution was filtered, 300ml of water and 200ml of ethyl acetate were added to the filtrate, the layers were separated, the organic layer was collected, concentrated, and passed through a silica gel chromatography column (gradient elution with dichloromethane:methanol=100:1 to 5:1), The product 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piper...
Embodiment 2
[0049] Example 2 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]phenyl]-propionic acid ethyl Synthesis of esters
[0050] 9.13g of 4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]bromobenzene was added to 400ml of acetonitrile, and 0.11 g bipyridine nickel bromide and 0.87g manganese dioxide (activated), add dropwise an acetonitrile solution containing 1.37g ethyl 2-chloropropionate at 50°C, and react at 50°C after the dropwise addition, HPLC method The reaction was stopped when the disappearance of one of the starting materials was detected.
[0051] The reaction solution was filtered, 400ml of water and 400ml of ethyl acetate were added to the filtrate, the layers were separated, the organic layer was collected, concentrated, and passed through a silica gel chromatography column (eluted with dichloromethane:methanol=100:1 to 5:1 gradient), The product 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]phenyl]-propionic...
Embodiment 3
[0054] Example 3 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]phenyl]-propionic acid iso Synthesis of Propyl Ester
[0055] Add 10.07g of 4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]-1-piperidinyl]ethyl]iodobenzene to 300ml ethylene glycol dimethyl In the ether, add 0.53g bipyridyl nickel bromide and 0.17g manganese dioxide (activated), add dropwise the ethylene glycol dimethyl ether solution containing 12.35g isopropyl 2-chloropropionate at 20°C, drop After the addition was completed, the reaction was incubated at 20°C, and the reaction was stopped after the disappearance of one of the raw materials was detected by HPLC.
[0056] The reaction solution was filtered, 200ml of water and 300ml of ethyl acetate were added to the filtrate, the layers were separated, the organic layer was collected, concentrated, and passed through a silica gel chromatography column (eluted with dichloromethane:methanol=100:1 to 5:1 gradient), The product 2-[4-[2-[4-[1-(2-etho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com