1,5,9-Triazapine compound and its synthesis method
A synthesis method and compound technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve the problems of unfavorable conjugated system derivation and expansion, harsh conditions, rare raw materials, etc., and achieve good thermal and chemical stability , mild reaction conditions and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0042] Example 2: Taking the synthesis of 4,8,12-tri-(p-methylphenyl)-1,5,9-triazapine as an example, its structural formula is as follows:
[0043]
[0044] The raw materials and synthesis methods used are:
[0045] In step 3 of this embodiment, benzoyl chloride is replaced with equimolar p-toluyl chloride, and other steps in this step are the same as those in embodiment 1. The other steps were the same as in Example 1, and the yellow solid 4,8,12-tris-(p-methylphenyl)-1,5,9-triazapine was prepared, the yield was: 73%, and the melting point was: > 300 o C.
[0046] The spectral data of the obtained product are as follows: 1 H NMR (500 MHz, CDCl 3 : CF 3 COOD = 0.5 mL : 10 μL): δ 9.64 (d, J = 9.1 Hz, 3H), 9.58 (d, J = 9.1 Hz, 3H), 8.12 (d, J = 7.7Hz, 6H), 7.70 (d, J = 7.65 Hz, 6H), 2.66 (s, 9H). 13 C NMR (125 MHz, CDCl 3 :CF 3 COOD = 0.5 mL : 10 μL): δ 163.5, 144.0, 141.0, 133.4, 131.7, 130.7, 130.0, 127.3, 126.1, 121.1, 115.1, 21.9. HRMS: m / z calcd for C ...
Embodiment 3
[0047] Example 3: Taking the synthesis of 4,8,12-tri-(p-methoxyphenyl)-1,5,9-triazapine as an example, its structural formula is as follows:
[0048]
[0049] The raw materials and synthesis methods used are:
[0050] In step 3 of this embodiment, benzoyl chloride is replaced with equimolar p-methoxybenzoyl chloride, and other steps in this step are the same as those in embodiment 1. The other steps were the same as in Example 1, and the yellow solid 4,8,12-tris-(p-methoxyphenyl)-1,5,9-triazapine was prepared, the yield was: 67%, and the melting point was :> 300 o C.
[0051] The spectral data of the obtained product are as follows:
[0052] 1 H NMR (500 MHz, CF 3 COOD): δ 9.82 (d, J = 9.1 Hz, 3H), 9.58 (d, J =9.1 Hz, 3H), 8.27 (d, J = 8.2 Hz, 6H), 7.49 (d, J = 8.3 Hz, 6H), 4.06 (s, 9H). 13 C NMR (125 MHz, CDCl 3 : CF 3 COOOD = 0.5 mL : 0.1 mL): δ 166.5, 164.0, 138.6, 136.9, 134.9, 128.6, 123.8, 121.8, 121.0, 117.0, 115.4, 56.4.
[0053] HRMS: m / z calcd for ...
Embodiment 4
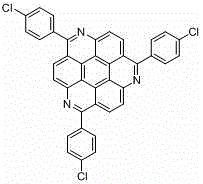
[0054] Example 4: Taking the synthesis of 4,8,12-tri-(p-chlorophenyl)-1,5,9-triazapine as an example, its structural formula is as follows:
[0055]
[0056] The raw materials and synthesis methods used are:
[0057] In step 3 of this embodiment, benzoyl chloride is replaced with equimolar p-chlorobenzoyl chloride, and in step 4, it is washed with dichloromethane, and other steps in this step are the same as those in embodiment 1. The other steps were the same as in Example 1, and 4,8,12-tris-(p-chlorophenyl)-1,5,9-triazapine was prepared as an earthy yellow solid, the yield was: 74%, and the melting point was: > 300 o C.
[0058] The spectral data of the obtained product are as follows: 1 H NMR (500 MHz, CDCl 3 : CF 3 COOD = 0.4 mL : 30 μL): δ 9.73 (d, J = 8.8 Hz, 3H), 9.69 (d, J = 8.6 Hz, 3H), 8.17 (d, J = 7.5Hz, 6H), 7.70 (d, J = 7.7 Hz, 6H). 13 C NMR (125 MHz, CDCl 3 : CF 3 COOD = 0.6 mL : 40 μL): δ 163.0, 141.7, 139.6, 134.8, 132.8, 130.7, 128.5, 127....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com