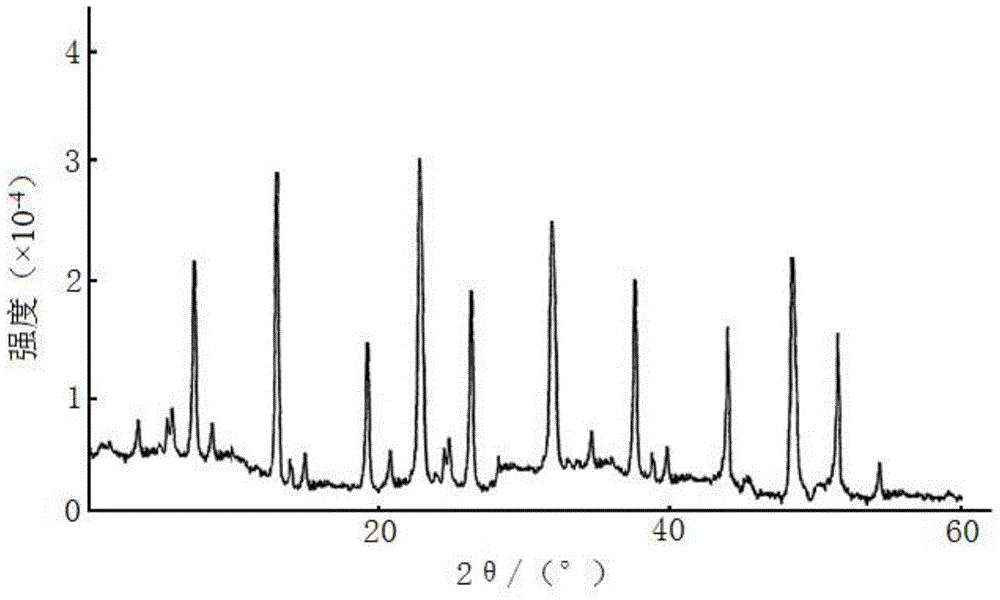
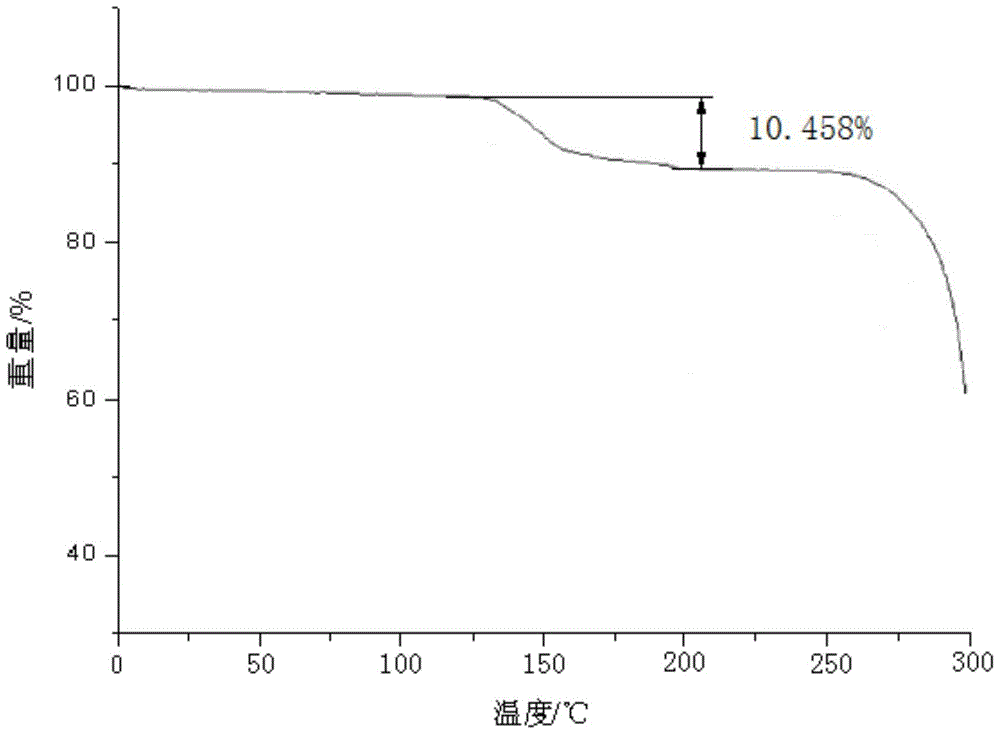
A kind of ceftezole sodium compound and the pharmaceutical preparation containing the compound
A technology of ceftezole sodium and pharmaceutical preparations, which is applied in the field of medicine, can solve the problems of quality stability, high polymer content, and small particle size of the product, and achieve the goal of reducing the risk of medication, good compatibility stability, and uniform particle size distribution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, the preparation of ceftezole sodium compound
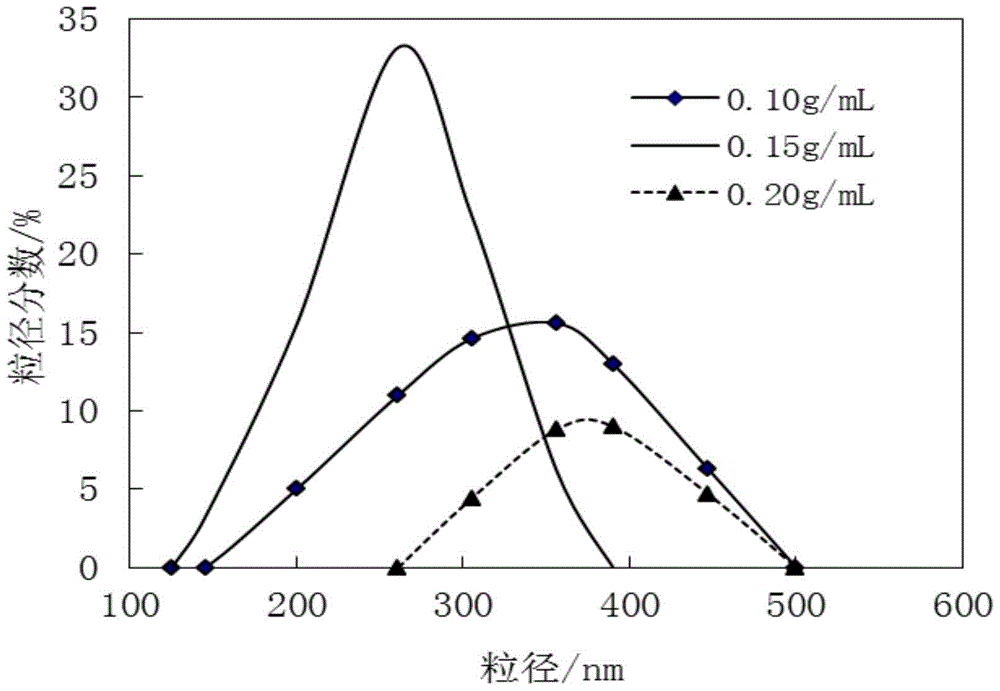
[0060] 1) 1.5 kg of ceftezole sodium was dissolved in 10 L of water to obtain a 0.15 g / mL aqueous solution of ceftezole sodium, which was filtered and the filtrate was used for subsequent use;
[0061] 2) At room temperature, add the filtrate described in step 1) into a mixed solution of 200L N,N-dimethylformamide and ethanol under stirring conditions, wherein the volume of N,N-dimethylformamide and ethanol The ratio was 1:3, and the stirring speed was 500r / min; after the filtrate was added, the temperature was lowered to 3°C, and the crystals were obtained, and the stirring was continued for 10 minutes; filtered, the filter cake was washed with water, and dried under reduced pressure to obtain the ceftezole sodium compound.
[0062] The ceftezole sodium compound of gained adopts U.S. Perkin-Elmer company PE 2400Ⅱ elemental analyzer, elemental analysis (%) calculated value is: C (30.23), H (3.29), N (21.71), N...
Embodiment 2
[0066] Embodiment 2, the preparation of ceftezole sodium compound
[0067] 1) 1.0 kg of ceftezole sodium was dissolved in 10 L of water to obtain a 0.10 g / mL aqueous solution of ceftezole sodium, filtered, and the filtrate was used for subsequent use;
[0068] 2) At room temperature, add the filtrate described in step 1) into the mixed solution of 100L N,N-dimethylformamide and ethanol under stirring conditions, wherein the volume of N,N-dimethylformamide and ethanol The ratio was 1:4, and the stirring speed was 600r / min; after the filtrate was added, the temperature was lowered to 0°C, and the crystal was obtained, and the stirring was continued for 8 minutes; filtered, the filter cake was washed with water, and dried under reduced pressure to obtain the ceftezole sodium compound.
[0069] The ceftezole sodium compound of gained adopts U.S. Perkin-Elmer company PE 2400Ⅱ elemental analyzer, elemental analysis (%) calculated value is: C (30.23), H (3.29), N (21.71), Na (4.46), ...
Embodiment 3
[0072] Embodiment 3, the preparation of ceftezole sodium compound
[0073]1) 1.2 kg of ceftezole sodium was dissolved in 10 L of water to obtain a 0.12 g / mL aqueous solution of ceftezole sodium, which was filtered and the filtrate was used for subsequent use;
[0074] 2) At room temperature, add the filtrate described in step 1) into a mixed solution of 150L N,N-dimethylformamide and ethanol under stirring conditions, wherein the volume of N,N-dimethylformamide and ethanol The ratio was 1:3.5, and the stirring speed was 550r / min; after the filtrate was added, the temperature was lowered to 5°C, and the crystals were obtained, and the stirring was continued for 12 minutes; filtered, the filter cake was washed with water, and dried under reduced pressure to obtain the ceftezole sodium compound.
[0075] The ceftezole sodium compound of gained adopts U.S. Perkin-Elmer company PE 2400Ⅱ elemental analyzer, elemental analysis (%) calculated value is: C (30.23), H (3.29), N (21.71), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com