Continuous c-di-AMP production method
A technology of c-di-amp and recombinant plasmids, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of few and unindustrialized applications, and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] In this example, taking Bacillus subtilis DisA as an example, the DisA-CMB gene recombination and recombinant expression plasmid construction, DisA-CMB protein expression and purification are briefly introduced as follows.
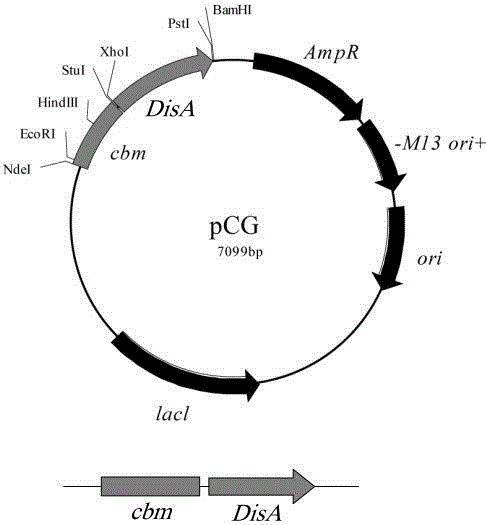
[0065] (1) DisA gene modification, by means of genetic engineering, the DisA gene and the cbm gene are fused to construct a recombinant plasmid vector; the schematic diagram of the pCG-DisA recombinant plasmid vector construction is as follows figure 2 Shown; specifically include the following steps:
[0066] First, primers are designed according to the known DisA gene sequence, and the corresponding DisA gene sequence is obtained by PCR amplification or whole-gene artificial synthesis technology; specifically, according to the sequence of the DisA gene of Bacillus subtilis and the structural characteristics of the pCG plasmid, upstream addition XhoI on the upper side, two restriction sites of BamHI on the lower side, the designed upstream and down...
Embodiment 2
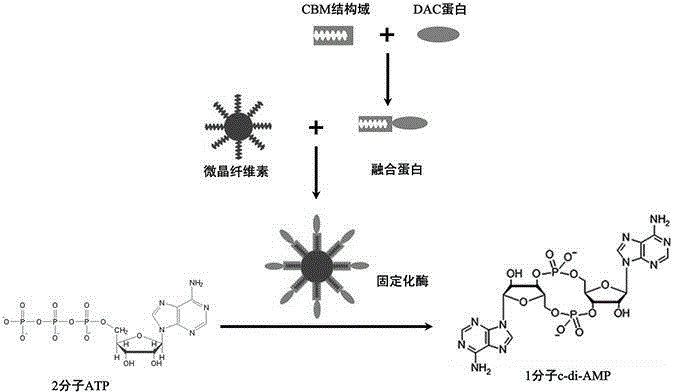
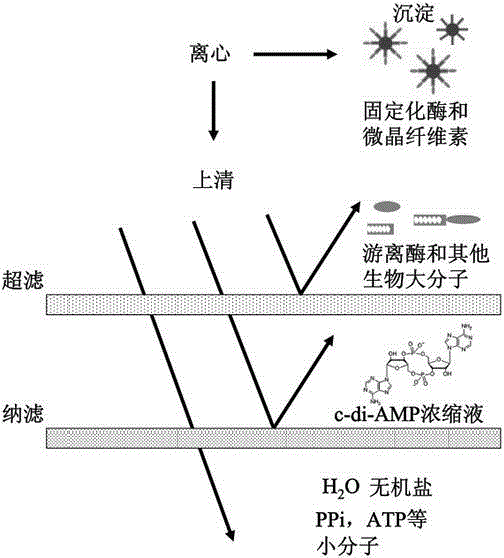
[0106] On the basis of Example 1, this example further explains the process of one-step purification and solidification of the CBM-DAC fusion protein, and the process of using the DisA protein to catalyze the synthesis of c-di-AMP and further purification, as follows.
[0107] (3) One-step purification and immobilization of CBM-DAC fusion protein; the specific steps are as follows:
[0108] First, the microcrystalline cellulose is preactivated and regenerated with phosphoric acid according to the method provided in the literature (Nat. Commun., 2014, 5, 3026); the preferred particle size of the microcrystalline cellulose is 10-100 μm. Specifically:
[0109] Weigh 100g microcrystalline cellulose (Aladdin, C104843-250g), dissolve in 0.3L deionized water, and mix well;
[0110] Add 5L of pre-cooled phosphoric acid, stir and mix evenly while adding, and clarification appears;
[0111] Place on ice for 1 hour, stir once every 10 minutes; then add 20L pre-cooled deionized water, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com