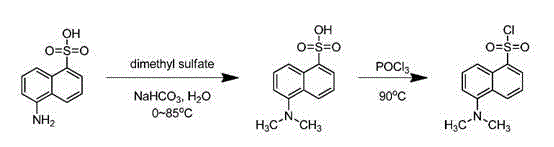
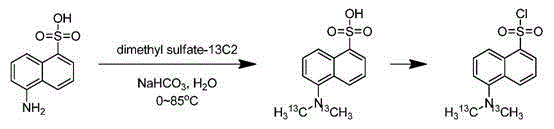
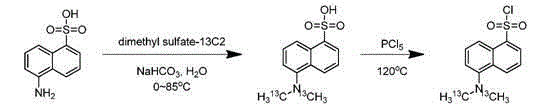
Synthesis method of isotope labeled dansyl chloride-13C2
An isotope labeling, dansyl chloride technology, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of large consumption of sodium bicarbonate, large environmental impact, and material flushing, and achieve simplified post-processing, improved safety, and safe operation. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Dissolve dansulphonic acid-13C2 (0.5g, 1.99mmol) in 20ml of anhydrous dichloromethane solvent, cool to 0°C under nitrogen protection, add dropwise anhydrous N,N-dimethylformamide (0.5ml, 22.56 mmol), stirred for 10 minutes, continued to dropwise add thionyl chloride (2ml, 27.54mmol), heated at 60°C for 4 hours after the dropwise addition; after the reaction, cooled the solution to room temperature, and added ice water while stirring the residue, And use sodium bicarbonate to adjust the pH value to 7, then extract twice with dichloromethane, combine all organic phases, use 10ml of dichloromethane each time, then wash with 5ml of water and 5ml of saturated brine successively, and wash the organic phase with 2 g of anhydrous magnesium sulfate was dried, filtered and concentrated to dryness to obtain 0.61 g of isotopically labeled dansyl chloride-13C2 crude product.
[0026] The isotope-labeled dansyl chloride-13C2 crude product obtained above was separated with a 400-mesh ...
Embodiment 2
[0029] Dissolve dansulphonic acid-13C2 (0.5g, 1.99mmol) in 20ml of anhydrous dichloromethane solvent, cool to 0°C under nitrogen protection, add dropwise anhydrous N,N-dimethylformamide (0.5ml, 22.56 mmol), stirred for 10 minutes, continued to add dropwise oxalyl chloride (2.4ml, 27.54mmol), heated at 60°C for 4 hours after the dropwise addition, after the reaction, cooled the solution to room temperature, and distilled off the solvent and chlorinated reagent under reduced pressure , add 10ml of ice water to the residue under stirring, adjust the pH value to 7 with sodium bicarbonate, extract the solution twice with diethyl ether, each time using 10ml of diethyl ether, combine all organic phases, then wash with 5ml of water and saturated with 5ml Wash with brine, dry the organic phase with 2 g of anhydrous magnesium sulfate, filter and concentrate to dryness to obtain 0.59 g of isotope-labeled dansyl chloride-13C2 crude product.
[0030] The isotope-labeled dansyl chloride-13C...
Embodiment 3
[0033]Dissolve dansulphonic acid-13C2 (0.5g, 1.99mmol) in 20ml of anhydrous tetrahydrofuran solvent, cool to 0°C under nitrogen protection, add dropwise anhydrous N,N-dimethylformamide (0.5ml, 22.56mmol) , stirred for 10 minutes, continued to add thionyl chloride (2ml, 27.54mmol) dropwise, and heated at 65°C for 4 hours after the dropwise addition was completed. After the reaction, the solution was cooled to room temperature, and the solvent and chlorinated reagent were distilled off under reduced pressure. 10ml of ice water was added to the residue under stirring, and the pH value was adjusted to 7 with sodium bicarbonate, and the solution was extracted twice with dichloromethane, using 10ml of dichloromethane each time, all organic phases were combined, and then washed with 5ml of water and Wash with 5 ml of saturated brine, dry the organic phase with 2 g of anhydrous magnesium sulfate, filter and concentrate to dryness to obtain 0.55 g of isotope-labeled dansyl chloride-13C2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com