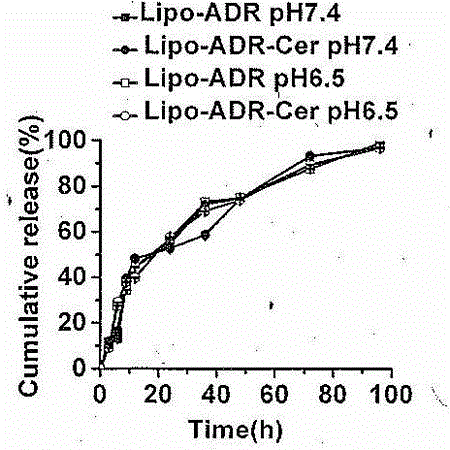
Drug-loading nano-liposome, and preparation method and application thereof
A nano-liposome and drug-carrying nanotechnology, which is applied in the application field of tumor treatment, can solve the problems of ceramide being difficult to mix with phospholipid molecules and poor treatment effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Implementation Example 1: Preparation of Lipo-ADR-Cer liposomes of the present invention and Lipo-ADR liposome blank liposomes
[0054] The composition and proportion of the blank liposome of PEG-Ceramide modified doxorubicin liposome: A hydrogenated soybean lecithin 24 mg (30.57nmol, 56.35%mol); B cholesterol 8mg (20.69nmol, 38.14%mol); C PEG2000-DSPE 2 mg (0.71 nmol, 1.31% mol); D PEG2000-C16-Ceramide 6 mg (2.28 nmol, 4.20%). PEG2000-DSPE and PEG2000-C16-Ceramide were purchased from Avanti Polar Lipids Company in the United States, cholesterol was purchased from Sigma Company, and hydrogenated soybean lecithin was purchased from Lipoid Company. The composition and ratio of the control doxorubicin liposome blank liposome: A hydrogenated soybean lecithin 24 mg (30.57nmol, 56.50%mol) B cholesterol 8mg (20.69nmol, 38.24%mol) C PEG2000-DSPE 8mg (2.85nmol ,5.27%mol). According to the above composition, accurately weigh the above ingredients into a 1.5 ml EP tube.
[00...
Embodiment 2
[0056] Implementation Example 2: Preparation of Lipo-ADR-Cer liposomes and Lipo-ADR liposome drug-loaded liposomes of the present invention
[0057]Take the liposome solution after dialysis in Example 1, add a pre-prepared 10 mg / ml doxorubicin solution (dissolved in MilliQ water, doxorubicin purchased from Dalian Meilun Biological Co., Ltd.), add 1ml blank liposome 75ul doxorubicin stock solution, the mixed solution during the drug loading process was placed in a water bath at 65°C for 15-20 min.
[0058] For removing the free doxorubicin of small molecules, the method of adopting dialysis is as follows: the prepared doxorubicin liposome solution is put into the dialysis bag that the molecular weight cut off is 1000KDa, and the dialysate is the HEPES solution (pH 7.4 of 1 L). ), the dialysis time is 6-8 hours, the fluid is changed twice, and the dialysis should be protected from light. Lipo-ADR-Cer liposomes and Lipo-ADR liposomes were obtained after dialysis, and were stor...
Embodiment 3
[0059] Implementation example 3: formulation optimization of co-loaded liposome Ceramide and ADR liposome
[0060] (1) The composition and ratio of C16-Ceramide co-loaded doxorubicin liposome blank liposomes: A Hydrogenated soybean lecithin 24 mg (30.57nmol, 56.35%mol) B Cholesterol 8mg (20.69nmol, 38.14%mol) C PEG2000-DSPE 2mg (0.71nmol, 1.31%mol) D C16-Ceramide 1.23mg (2.28nmol, 4.20%). PEG2000—DSPE and C16-Ceramide were purchased from Avanti Polar Lipids Company in the United States, cholesterol was purchased from Sigma Company, and hydrogenated soybean lecithin was purchased from Lipoid Company. The preparation process of liposomes co-loading C16-Ceramide and doxorubicin is as in Example 2.
[0061] (2) The composition and proportion of the blank liposomes co-loaded with doxorubicin liposomes by C6-Ceramide: hydrogenated soybean lecithin 24 mg (30.57 nmol, 56.35% mol) B cholesterol 8 mg (20.69 nmol, 38.14% mol) C PEG2000-DSPE 2mg (0.71nmol, 1.31%mol) D C6-Ceramide 0.91...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com