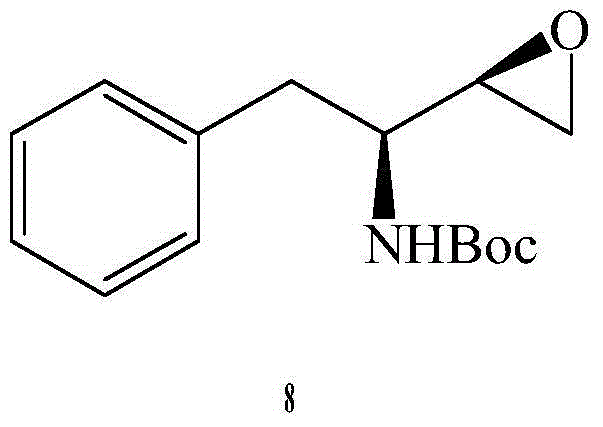
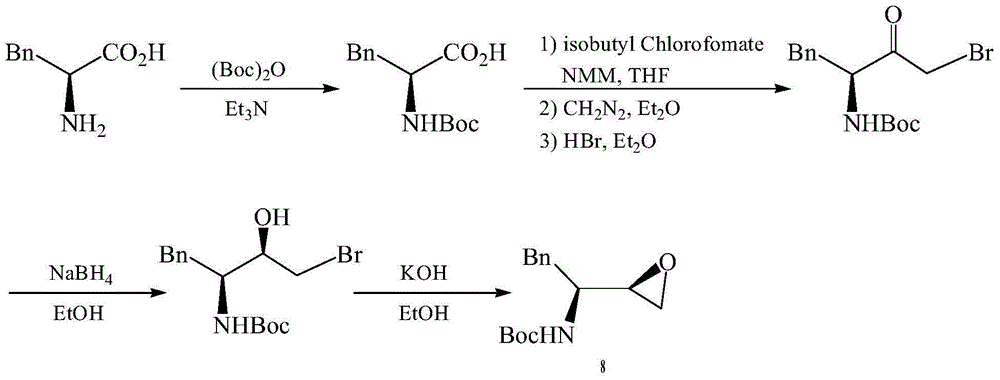
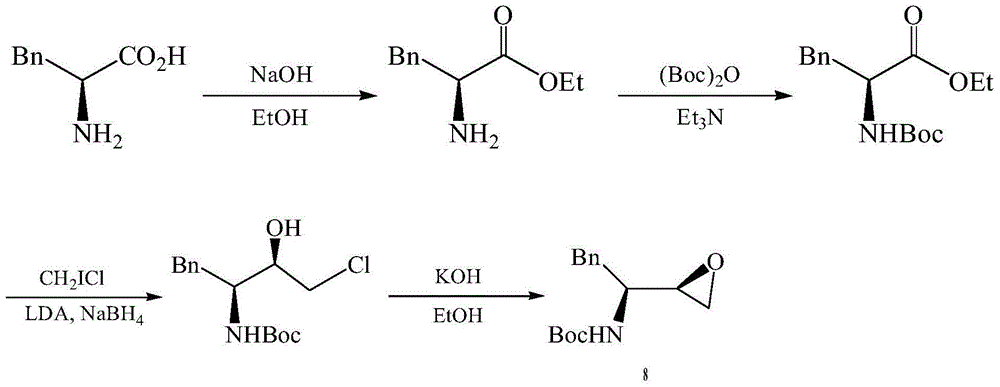
Preparation method of darunavir intermediate
A technology of darunavir and intermediates, applied in the direction of organic chemistry and the like, can solve the problems of low safety, difficult separation, complicated process and the like, and achieve the effects of reasonable process, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Dissolve L-phenylalanine 1 (85g, 0.515mol) in methanol (250ml), and add thionyl chloride (182.1g, 1.545mol) dropwise under a controlled temperature of 0°C under nitrogen atmosphere , control the reaction temperature at 30°C, and stir the reaction for 12h. After the reaction, it was extracted with ethyl acetate (2×150ml) and sodium bicarbonate (2×150ml), dried, filtered, and concentrated under reduced pressure to obtain white solid 2 (84.8g, 92%). Mp:158-162℃.IR(KBr):3374,3028,2952,1732,1538,1263cm -1 . 1H NMR (300MHz, CDCl 3 ):δ7.42-7.30(m,5H,ArH),4.80-4.60(t,1H,J=7.0Hz,CH),3.83(s,3H,OCH 3 ),3.33-3.23(dd,2H,J1=J2=5.12Hz,CH 2 ) ppm. 13 C NMR (75MHz, CDCl 3 ): δ169,133,129,128,127,58,54,36ppm.
[0047] (2) The mixture of L-phenylalanine methyl ester 2 (84.8g, 0.474mol), benzyl chloride (179.2g, 1.422mol) and tetrahydrofuran (250ml) was reacted at room temperature for 10h. After the reaction, it was basified with potassium carbonate (2×150ml), extracted, dried,...
Embodiment 2
[0054] (1) Dissolve L-phenylalanine 1 (85g, 0.515mol) in methanol (250ml), and add thionyl chloride (212.1g, 1.8mol) dropwise under a controlled temperature of 0°C under nitrogen atmosphere , control the reaction temperature at 40°C, and stir the reaction for 13h. After the reaction, it was extracted with ethyl acetate (2×150ml) and sodium bicarbonate (2×150ml), dried, filtered, and concentrated under reduced pressure to obtain white solid 2 (85.4g, 92.6%).
[0055] (2) The mixture of L-phenylalanine methyl ester 2 (85.4g, 0.476mol), benzyl chloride (210.4g, 1.67mol) and tetrahydrofuran (250ml) was reacted at room temperature for 11h. After the reaction, it was basified with potassium carbonate (2×150ml), extracted, dried, filtered, concentrated under reduced pressure, column chromatography (ethyl acetate:hexane=1:50→1:5) to obtain a colorless oily liquid 3 (163.8 g, 95.8%).
[0056] (3) N, N-dibenzyl-L-phenylalanine methyl ester 3 (163.8g, 0.456mol), bromochloromethane (87....
Embodiment 3
[0062] (1) Dissolve L-phenylalanine 1 (85g, 0.515mol) in methanol (250ml), and add thionyl chloride (242.9g, 2.06mol) dropwise under a controlled temperature of 0°C under nitrogen atmosphere , control the reaction temperature at 50°C, and stir the reaction for 14h. After the reaction, it was extracted with ethyl acetate (2×150ml) and sodium bicarbonate (2×150ml), dried, filtered, and concentrated under reduced pressure to obtain white solid 2 (86.7g, 94%).
[0063] (2) The mixture of L-phenylalanine methyl ester 2 (86.7g, 0.484mol), benzyl chloride (244g, 1.936mol) and tetrahydrofuran (250ml) was reacted at room temperature for 12h. After the reaction, it was basified with potassium carbonate (2×150ml), extracted, dried, filtered, concentrated under reduced pressure, column chromatography (ethyl acetate:hexane=1:50→1:5) to obtain a colorless oily liquid 3 (167.4g, 96.3%).
[0064] (3) N, N-dibenzyl-L-phenylalanine methyl ester 3 (167.4g, 0.466mol), bromochloromethane (119.2g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com