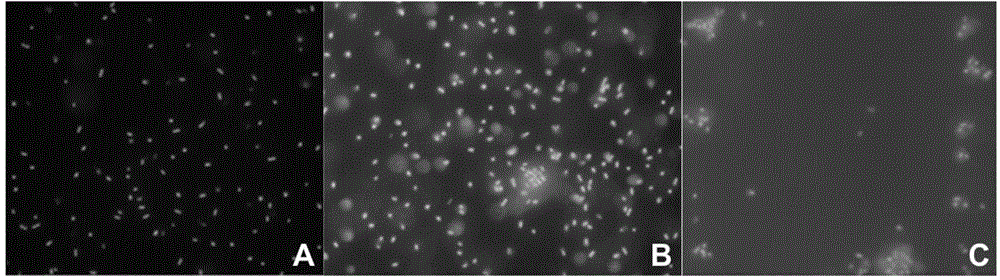
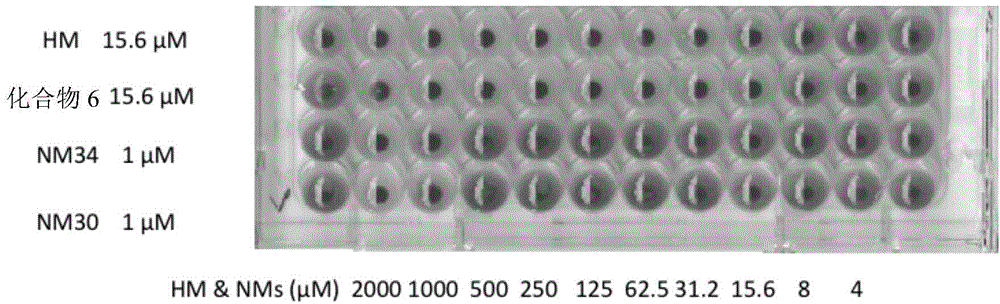
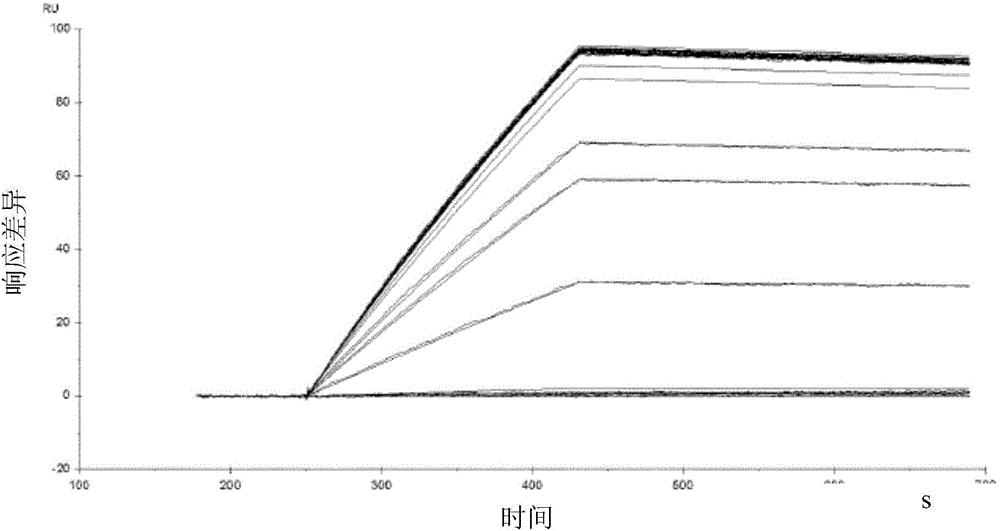
Multimeric mannosides, a process for preparing the same and their uses as a drug
A technology of compounds and derivatives, applied in the fields of polymannosides and their preparation and their use as medicines, can solve the problems of not easily adapting to pharmacological properties, permeability, affinity and retention, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1384] Example 1: Synthesis of Heptylmannoside Cyclodextrin Compound 2
[1385]
[1386] 8-oxa-undec-10-ynyl 2,3,4,6-tetra-O-acetyl-α-D-mannopyranoside 9’
[1387] Mannosyl pentaacetate (229 mg, 0.587 mmol), compound 8' (150 mg, 0.882 mmol) and silver trifluoroacetate (194 mg, 0.878 mmol) were dissolved in anhydrous dichloromethane (3 mL). Add 1M SnCl 4 Solution in dichloromethane (585 μL), under argon atmosphere, the mixture was stirred at room temperature for 3 hours. The solution was diluted in dichloromethane (10 mL), washed with saturated NaHCO 3 (2 x 10 mL) washes. The organic layer was dried, filtered and evaporated under reduced pressure. The residue was chromatographed on silica gel using ethyl acetate-cyclohexane (2-8) to (3-7) to afford 9' (128 mg, 44%) as a colorless oil. Analytical data were the same as previously described [Gouin, S.G.; Wellens, A.; Bouckaert, J.; Kovensky, J. Chem Med Chem. 2009, 5, 749-755].
[1388] 8-oxa-undec-10-ynyl-α-D-mannopyrano...
Embodiment 2
[1395] Example 2: Synthesis of Heptylmannoside Cyclodextrin Compound 4
[1396] - Compound 16' of the formula:
[1397]
[1398] Compound 15' (112 mg, 423 μmol) of the following formula:
[1399]
[1400] and hepta-6-azido-6-deoxy-β-cyclodextrin (60 mg, 47 μmol) were dissolved in DMF / H 2 O mixture (5 / 1.6mL). Copper sulfate (15 mg, 94 μmol) and sodium ascorbate (37 mg, 187 μmol) were added, and the mixture was stirred at room temperature for 19 hours. The mixture was evaporated under reduced pressure, and the residue was dissolved in DMF (15 mL) together with sodium azide (121 mg, 1.86 mmol). The mixture was stirred at 70°C for 36 hours. The mixture was evaporated under reduced pressure and the residue was purified by preparative HPLC to give 16' (21 mg, 15%) as a white powder after lyophilization.
[1401] [α] D =+84 (c=0.1, H 2 0); Tr=36 minutes; 1 H NMR (500MHz, DMSO) δ = 7.95 (7H, s, H 三唑 ), 6.00, 5.88 (14H, br, OH), 5.08 (7H, br, H-1 I-VII ), 4.50-4.00 (36H...
Embodiment 3
[1437] Example 3: Synthesis of 5-acetyl-2-((α-D-mannopyranosyl)amino)thiazole[6]
[1438] 5-Acetyl-2-((2,3,4,6-tetra-O-acetyl-α-D-mannopyranosyl)amino)thiazole[6a]
[1439]
[1440] α and β ratio (9 / 1):
[1441] 1 H NMR (300MHz, CDCl 3 )δ=9.00(br, 1H, NH), 7.98(s, 0.9H, H8 α ), 7.79(s, 0.1H, CH8 β ), 5.52 (dd, J 2,1 = 1.8Hz,J 2,3 =2.7Hz, 1H, H2), 5.34-5.24(m, 2H, H4 and H3), 5.14(d, 1H, J 1,2 = 1.8Hz, H1 α ), 4.32 (dd, 1H, J 6b,6a =12.0Hz,J 6b,5 =5.4Hz, H6 b ), 4.11-4.01 (m, 2H, H6 a and H5), 2.43 (s, 3H, H11), 2.18, 2.06, 2.01, 2.00 (4s, 12H, CH 3 CO).
[1442] 13 C NMR (300MHz, CDCl 3 )δ=189.8 (C10), 173.1 (C7), 170.8, 170.2, 169.7 (4CH 3 CO), 146.7(C8), 131.5(C9), 82.5(C1), 69.4(C5+C3 or C4), 69.1(C2), 66.0(C3 or C4), 62.2(C6), 26.2(C11), 20.7 (4CH 3 CO).
[1443] MS(CI)m / z=473[M+H] + .
[1444] HRMS (MALDI): calculated value C 19 h 24 N 2 o 10 SH[M+H] +473.1224; found 473.1241
[1445] [a] 26 D +87(c 0.175, CHCl 3 )
[1446] 5-Acetyl-2-((α...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com