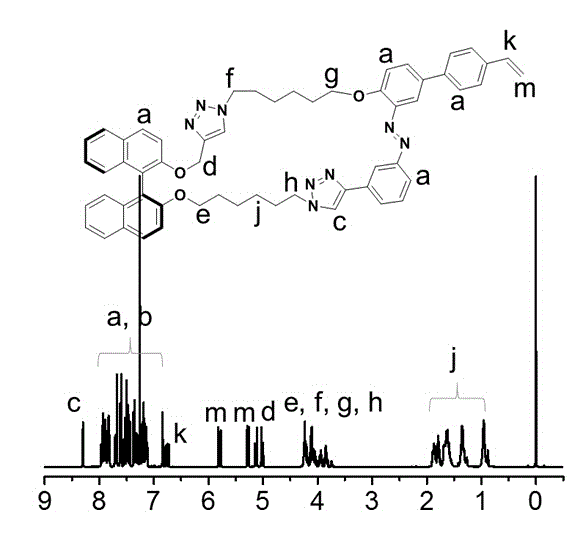
Polymer containing cyclic azobenzene-dinaphthalene structure on side chain as well as preparation method and application of polymer
A technology of cyclic azobenzene and polymers, which is applied in the field of chiral optical polymers and can solve problems such as blanks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Synthesis of azobenzene donor.
[0093] 1. Synthesis of azophenol intermediate (compound 1).
[0094] Add 18.8g (0.16mol) m-aminophenylacetylene, 100mL deionized water and 40.5mL concentrated hydrochloric acid into a 500mL beaker, and stir in an ice-salt bath. When the temperature drops to 0~5°C, add dropwise an aqueous solution of sodium nitrite (prepared by dissolving 12.35g (0.18mol) of sodium nitrite in 100mL of deionized water), keep the system temperature at 0~5°C, and complete the dropwise addition Afterwards, the reaction was continued for 20 minutes to obtain a diazonium salt solution.
[0095] Add 41.72g (0.24mol) of p-bromophenol, 300mL of deionized water and 12.85g (0.32mol) of sodium hydroxide into a 1000mL beaker, and stir in an ice-water bath. When the temperature drops to 0-5°C, add sodium bicarbonate to adjust the pH value to 9. The above-mentioned diazonium salt solution was slowly dropped into the system, and the reaction was continued f...
Embodiment 2
[0100] Example 2: Synthesis of binaphthyl donor.
[0101] 1. Synthesis of brominated binaphthyl intermediate (compound 3).
[0102] Add 2.9g (21mmol) of potassium carbonate and a catalytic amount of potassium iodide to a 1000mL three-necked flask, and dissolve them fully with 700mL of acetone. 2.86g (10mmol) of 2,2'-binaphthol was added under stirring, and while the temperature was raised, 2.66g (11mmol) of 1,6-dibromohexane in acetone was added dropwise, and refluxed at 80°C for 100 minutes. TLC tracking (the ratio of developer is n-hexane: ethyl acetate = 10:1) shows that by-products are formed in about 1.5 hours, and the heating should be stopped at this time. Natural cooling at room temperature, precipitation, rotary evaporation of acetone solution, purification by column chromatography with a mixed solvent of n-hexane and ethyl acetate (the ratio of developing solvent is n-hexane: ethyl acetate = 10:1), and spin-dried to obtain 1.87g target product.
[0103]
[0104...
Embodiment 3
[0110] Example 3: CuAAC ring-closing reaction between two donors.
[0111] 1. Synthesis of compound 6 (the first CuAAC reaction between an alkyne group and an azide group for linking two donors).
[0112] Take 0.2g (0.3mmol) of compound 5 and 0.16g (0.3mmol) of compound 2 into a three-necked flask, and then add 5mL of dichloromethane into it to dissolve. After passing argon for 30 minutes, 1 mg (7 μmol) of cuprous bromide and 6 ml (7 μmol) of pentamethyldiethylenetriamine (PMDETA) were added, and refluxed at 30°C for 40 minutes in an argon atmosphere. After the solvent was evaporated to dryness in vacuo, 0.30 g of the target product (compound 6) was obtained by column chromatography (developing solvent ratio: n-hexane: ethyl acetate = 4:1).
[0113]
[0114] 2. Synthesis of compound 7 (azidation reaction, used to introduce another azido group).
[0115] The process of compound 7 synthesized from compound 6 through azidation reaction can refer to the synthesis process of c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com