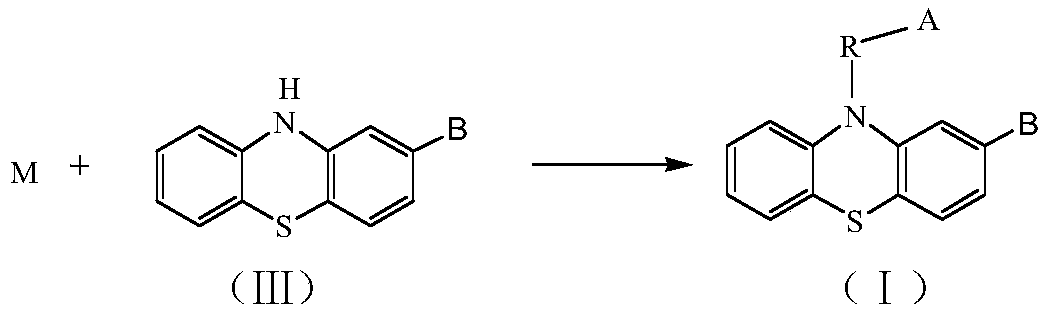
Phenothiazines and their preparation methods and applications
A technology of phenothiazines and compounds, applied in the field of anticancer drugs, can solve the problems of tumor recurrence and metastasis, and achieve the effects of high yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation 2-21-2
[0029]
[0030] 2-10:
[0031] Take 2-trifluoromethylphenothiazine (2.00g, 7.4831mmol) and place it in a 500mL flask, add 2 CO 3 Dry THF (100mL), then in N 2 Under protection, 60% NaH mineral oil (1.1973 g, 29.9323 mmol) and 1-bromo-3-chloropropane (2.9601 mL, 29.9323 mmol) were sequentially added, and the mixture was refluxed overnight at a bath temperature of 65°C. Cool to room temperature, then pour the reaction solution into an ice-water mixture, extract with ethyl acetate (300mL+100mL×2), combine the organic phases, wash with anhydrous MgSO 4 Dry, filter to remove the solid, then spin the filtrate to dryness, add ethyl acetate (50mL) to dissolve, add silica gel powder (20g) to the solution and spin dry, dry load the sample through a silica gel column for purification, use ethyl acetate and petroleum ether After gradient elution, the product was collected, spin-dried, and vacuum-dried to obtain product 2-10 (4.9444 g, yield 69....
Embodiment 2
[0034] Example 2: Preparation 2-21-6
[0035]
[0036] In a 30mL straight bottle, add 2-10 (250mg, 0.7272mmol), prolinol (110.3mg, 1.0908mmol), triethylamine (0.5mL) and acetonitrile (10mL), and stir overnight at 80°C. Cool to room temperature, spin dry directly, dissolve the crude product in methanol, filter, add silica gel powder to the filtrate to make a solid solution, dry load the sample through a silica gel column for purification, and shower with triethylamine, ethyl acetate and petroleum ether gradient After washing, the product was collected, spin-dried, and vacuum-dried to obtain product 2-21-6 (138.9 mg, yield 38.9%). 1 H-NMR (400MHz, CDCl 3 )δ7.25-7.13(m,4H),7.05(s,1H),7.01-6.89(m,2H),4.05-3.96(m,2H),3.68-3.60(m,1H),3.51-3.42( m,1H),3.33-3.23(m,1H),3.14-3.03(m,1H),2.81-2.69(m,1H),2.61-2.50(m,1H),2.35-2.24(m,1H), 2.16-2.05(m,2H),1.85-1.72(m,3H),1.28-1.23(m,2H);+c ESI Q1MS: [M+H +] 409.11.
Embodiment 3
[0037] Example 3: Preparation of 2-163, 2-172
[0038]
[0039] 2-21-1:
[0040] Take 2-10 (300mg, 0.8726mmol) and place it in a 30mL straight bottle, then add 1-tert-butoxycarbonylpiperazine (243.8mg, 1.3089mmol), triethylamine (0.37ml, 2.6178mmol) and acetonitrile (10mL) , react overnight at 80°C. Cool to room temperature, directly evaporate to dryness, dissolve the crude product in methanol, then add silica gel powder to the filtrate to make a solid solution, dry load the sample through a silica gel column for purification, and use gradient elution with ethyl acetate, petroleum ether and triethylamine , collected the product, spin-dried, and vacuum-dried to obtain the product 2-21-1 (63.4 mg, yield 40.2%). 1 H-NMR (400MHz, CDCl 3 )δ7.24-7.10(m,5H),7.04(s,1H),7.00-6.88(m,1H),4.08-3.95(m,2H),3.60-3.25(m,4H),2.70-2.25( m,6H),2.12-1.90(m,2H),1.44(s,9H);+c ESI Q1MS: [M+H + ] 494.20.
[0041] 2-163:
[0042] In a 100 mL flask, 2-21-1 (2.4458 g, 4.9569 mmol) was dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com