Targeted tumor associated fibroblast liposome drug delivery system
A technology related to fibroblasts and tumors, applied in the field of new tumor-associated fibroblasts targeting drug-loaded liposomes and liposome drug-loaded systems, can solve the problem of weakening the penetration of nano-preparations, increasing interstitial pressure, To improve the tumor microenvironment, reduce toxicity and enhance the efficacy of anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
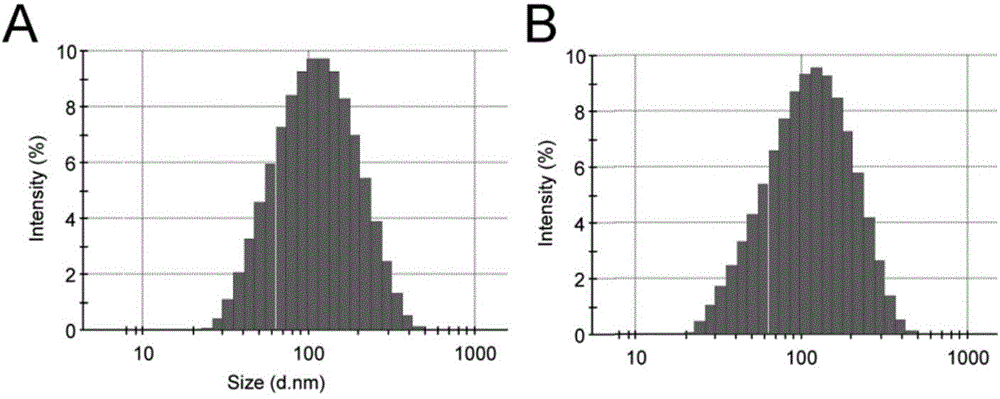
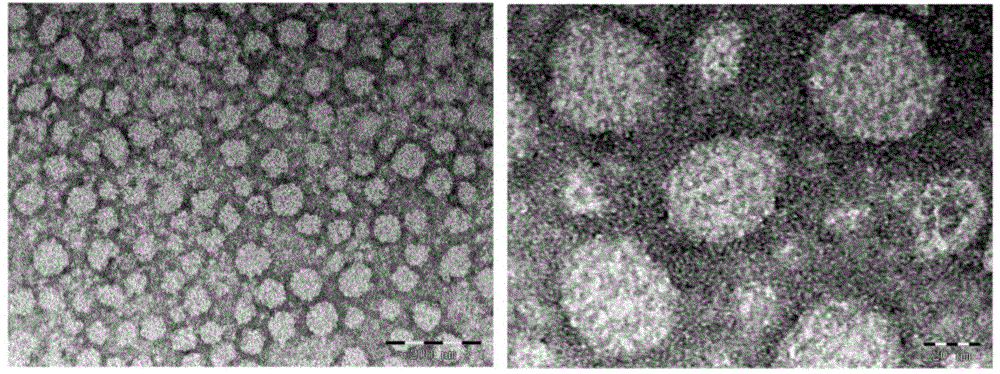
[0043] Example 1. Preparation of drug-loaded liposomes targeting tumor-associated fibroblasts
[0044] 1. Synthesis of Guided Compounds
[0045] A certain amount of NHS active esterified polyethylene glycol-modified phospholipid and polypeptide powder (molar ratio 2:1) was weighed and dissolved in anhydrous dimethylformamide respectively. After the powder is completely dissolved, the polypeptide solution is first transferred to an eggplant-shaped bottle, and the NHS active esterified polyethylene glycol-modified phospholipid solution is added dropwise to the polypeptide solution under magnetic stirring. After mixing evenly, add an appropriate amount of triethylamine to adjust the pH of the reaction solution to 8.0-9.0, and react at room temperature for 96 hours under the protection of nitrogen and light. During the reaction, the progress of the reaction was followed by thin layer chromatography. At the end of the reaction, the target compound was obtained by dialysis and fre...
Embodiment 2
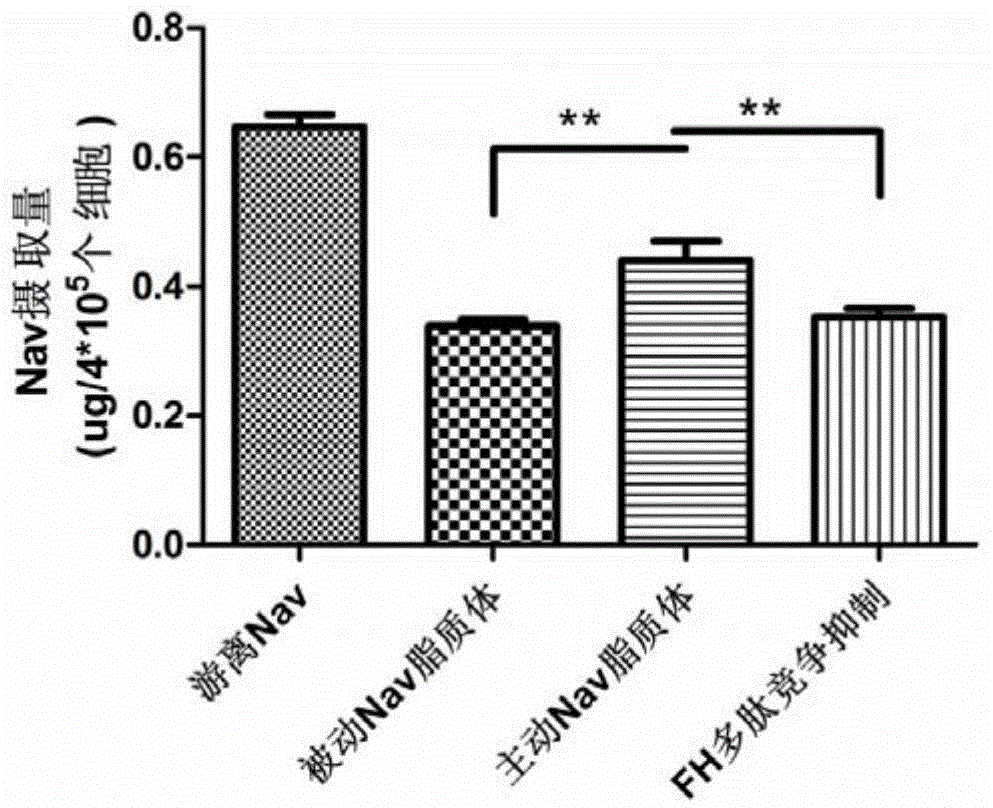
[0059] Example 2. Targeting evaluation of FH polypeptide-liposome / Navitoclax on tumor-associated fibroblasts in vitro
[0060] HPLC was used to measure the uptake of free Navitoclax, liposome / Navitoclax, FH polypeptide-liposome / Navitoclax and FH polypeptide+FH polypeptide-liposome / Navitoclax by LX‐2 cells. LX‐2 cells were seeded in 24-well plates, incubated overnight until they were completely attached to the wall, discarded the original culture medium, and washed with phosphate buffer; 1ml of different Navitoclax preparations (free Navitoclax, liposome / Navitoclax, FH polypeptide‐liposome / Navitoclax and FH polypeptide+FH polypeptide‐liposome / Navitoclax), the final concentration of Navitoclax was 40 μg / ml, and incubated in a 37°C incubator for 3 hours; Phosphate buffer solution to stop cell uptake, then add 0.2ml RIPA cell lysate, lyse the cells at room temperature for 5min; transfer the cell lysate to a 1.5ml EP tube, add 0.3ml methanol to each well, and transfer to Correspo...
Embodiment 3
[0061] Establish a nude mouse model bearing Hep G2 tumor until the tumor volume is 200mm 3 , were randomly divided into 4 groups, and injected 200 μl of normal saline, free Navitoclax, liposome / Navitoclax and FH polypeptide-liposome / Navitoclax respectively into the tail vein. The dose of Navitoclax is 5 mg / kg, administered every other day, for a total of three administrations. The animals were sacrificed on the third day after the third administration, and frozen sections of tumor tissues were prepared. Tumor-associated fibroblasts were labeled by immunofluorescence: the frozen sections of the tumor were dried by cold air, fixed with 4% paraformaldehyde at room temperature for 15 minutes, washed with phosphate buffer for 3 times, each time for 5 minutes, and placed on glass after cleaning. Mark the tumor area on the back of the slice; add 0.1% TritonX‐100 (prepared in phosphate buffer), permeabilize at room temperature for 5 minutes, wash with a large amount of PBS again for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com