Preparing method of (E)-N'-arylmethylene-4-(coumarin-3-yl)thiazole-2-hydrazide compound and its application
A kind of technology of aryl methylene and compound, applied in (E)-N'-aryl methylene-4-(coumarin-3-yl)thiazole-2-hydrazide compound preparation method and application It can solve the problems of difficulty in the treatment of infectious diseases and achieve good inhibitory and killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
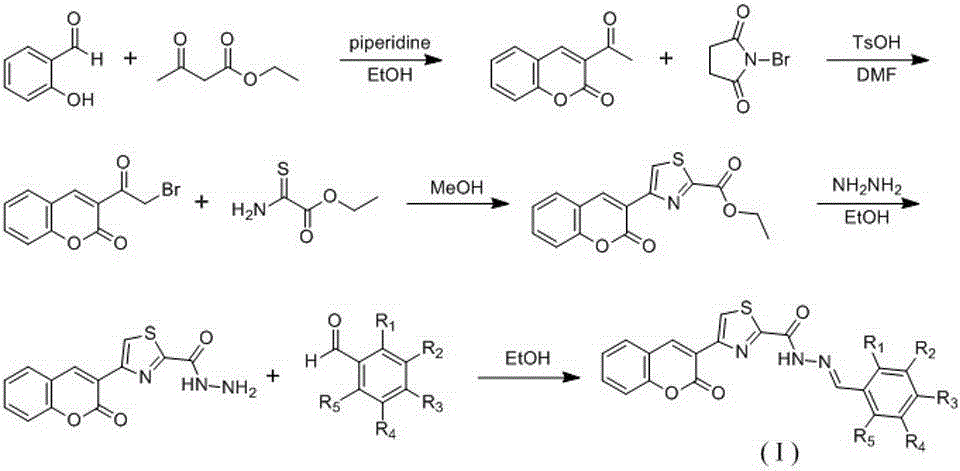
[0023] Example 1: Preparation of 3-acetyl-2H-benzopyran-2-one
[0024]
[0025] Put salicylaldehyde (1.22 g, 10 mmol), ethyl acetoacetate (2.6 g, 20 mmol), and piperidine (0.5 mL) in a round-bottomed flask, add 100 mL of ethanol, reflux for 12 hours, TLC shows the reaction After completion, stop the reaction, cool to room temperature, add 300 mL of water, extract with ethyl acetate (200 mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, spin dry, and separate and purify through a silica gel column to obtain a yellow solid powder 1.5 g, yield 79%. 1 H NMR (CDCl 3 , 400 MHz) δ: 2.73 (s, 3H), 7.33-7.35 (m, 2H), 7.64-7.69 (m, 2H), 8.51 (s, 1H).
Embodiment 2
[0026] Embodiment two: the preparation of 3-(2-bromoacetyl)-2H-benzopyran-2-one
[0027]
[0028] 3-Acetyl-2H-benzopyran-2-one (188 mg, 1 mmol), p-toluenesulfonic acid (380 mg, 2 mmol), N-bromosuccinimide (354 mg, 2 mmol) was placed in a round-bottomed flask, 10 mL of DMF was added, and the reaction was carried out at 100 °C for 2 hours. TLC showed that the reaction was complete, and the reaction was stopped. Saturated sodium thiosulfate solution was added, extracted with ethyl acetate, the organic phases were combined, and purified by silica gel column chromatography , to obtain 127 mg of white solid powder with a yield of 44%. 1 H NMR (CDCl 3 , 400 MHz) δ: 4.76 (s, 2H), 7.37-7.42 (m, 2H), 7.69-7.73 (m, 2H), 8.65 (s, 1H).
Embodiment 3
[0029] Example 3: Preparation of ethyl 4-(2-oxo-2H-benzopyran-3-yl)thiazole-2-carboxylate
[0030]
[0031] 3-(2-Bromoacetyl)-2H-benzopyran-2-one (133 mg, 1 mmol), thiooxamide ethyl ester (399 mg, 3 mmol) were placed in a round bottom flask and added 10 mL of methanol was stirred at room temperature for 24 hours, TLC showed that the reaction was complete, the reaction was stopped, 20 mL of water was added, extracted with ethyl acetate, the organic phases were combined, and purified by silica gel column chromatography to obtain 190 mg of yellow-brown solid powder with a yield of 63%. 1 H NMR (CDCl 3 , 400 MHz) δ: 1.49 (t, 3H, J = 7.2 Hz), 4.51 (q, 2H, J = 7.2 Hz), 7.32 (t, 1H, J = 8.0 Hz), 7.38 (d, 1H, J = 7.2 Hz) 8.0 Hz), 7.58 (t, 1H, J = 8.0 Hz), 7.66 (d, 1H, J = 8.0 Hz), 8.74 (s, 1H), 8.92 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com