Method for preparing 2-nitro-4-methylsulfonylbenzoic acid through indirect electro-oxidation
A technology of thiamphenicol benzoic acid and thiamphenicol toluene, which is applied in the field of indirect electrooxidation to prepare o-nitro-p-thiamphenicol benzoic acid, can solve the problems of easy decomposition, weak hydrogen peroxide oxidation ability, and large amount of hydrogen peroxide, and avoid Environmental pollution problems, oxidant cost savings, and high raw material utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Indirect Electrooxidation Preparation of o-nitro-p-thiamphenicol Benzoic Acid
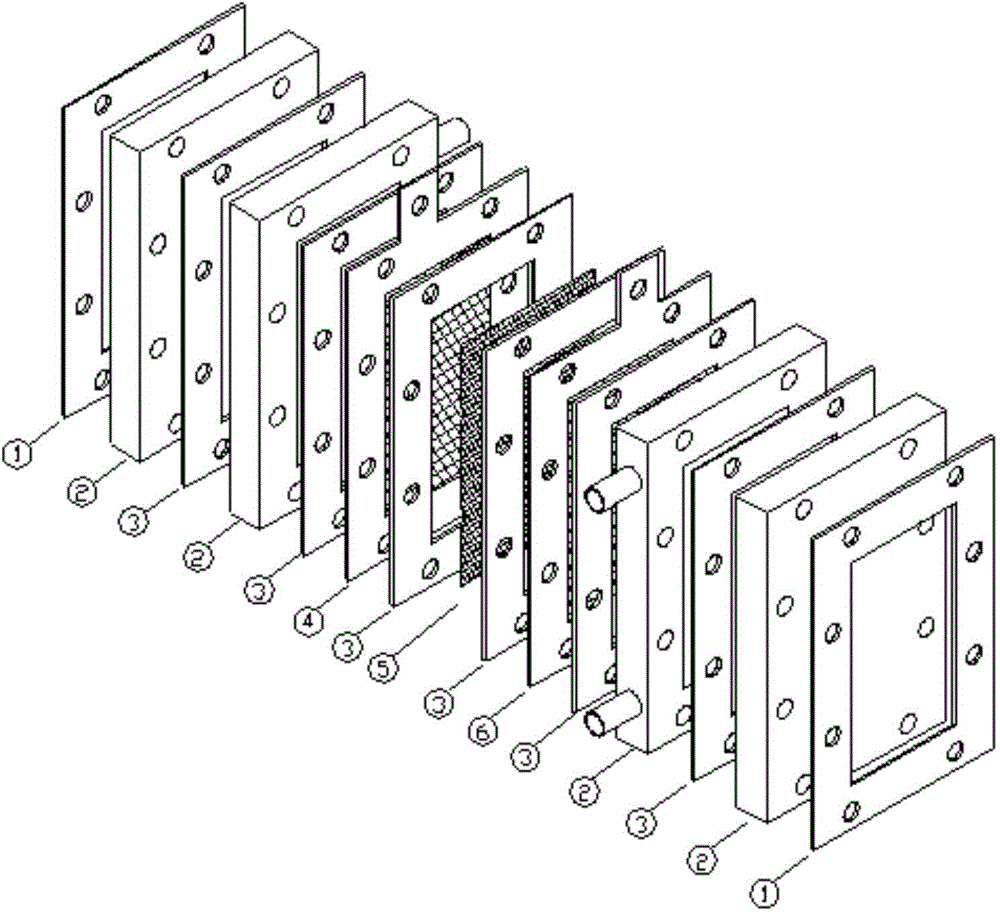
[0020] In the 250ml reactor, add the sulfuric acid of 43g concentration 6mol / L, 4g chromium trioxide, the reactor is connected with stirrer, thermometer, condenser. Start stirring and heating. When the temperature rises to 90°C, add 4.3g of o-nitro-p-thiamphenicol toluene, and react at a constant temperature at this temperature for 6 hours. Cool the reaction solution to below 30°C, filter and wash and dry to obtain a solid of 3.81 g, The yield of o-nitro-p-thiamphenicol benzoic acid is 38.68%. The filtrate after suction filtration is Cr 3+ mother liquor. Titration of Cr in Mother Liquor by Ferrous Ammonium Sulfate Titration 6+ After content, the mother liquor is sent to the electrolytic cell for electrolysis. The electrolytic cell is a self-made plate-and-frame electrolytic cell. The length-to-height ratio of the cathode and anode chambers is 6:1:9. The membrane used is a cation exchange...
Embodiment 2
[0022] Indirect Electrooxidation Preparation of o-nitro-p-thiamphenicol Benzoic Acid
[0023] In the 250ml reactor, add the sulfuric acid of 43g concentration 7mol / L, 8g chromium trioxide, the reactor is connected with stirrer, thermometer, condenser. Start stirring and heating. When the temperature rises to 90°C, add 4.3g of o-nitro-p-thiamphenicol toluene, and react at this temperature for 6 hours. g, the yield of o-nitro-p-thiamphenicol benzoic acid was 42.65%. The filtrate after suction filtration is Cr 3+ mother liquor. Titration of Cr in Mother Liquor by Ferrous Ammonium Sulfate Titration 6+ After content, the mother liquor is sent to the electrolytic cell for electrolysis. The electrolytic cell is a self-made plate-and-frame electrolytic cell. The length-to-height ratio of the cathode and anode chambers is 6:1:9. The membrane used is a cation exchange membrane, and the cathode and anode are Pb / PbO respectively. 2 plate and stainless steel plate, the catholyte is 7m...
Embodiment 3
[0025] Indirect Electrooxidation Preparation of o-nitro-p-thiamphenicol Benzoic Acid
[0026] In the 250ml reactor, add the sulfuric acid of 103.2g concentration 7mol / L, 16g chromium trioxide, the reactor is connected with stirrer, thermometer, condenser. Start stirring and heating. When the temperature rises to 90°C, add 17.2g of o-nitro-p-thiamphenicol toluene, and react at a constant temperature for 6 hours at this temperature. g, the yield of o-nitro-p-thiamphenicol benzoic acid is 43.28%. The filtrate after suction filtration is Cr 3+ mother liquor. Titration of Cr in Mother Liquor by Ferrous Ammonium Sulfate Titration 6+ After content, the mother liquor is sent to the electrolytic cell for electrolysis. The electrolytic cell is a self-made plate-and-frame electrolytic cell. The length-to-height ratio of the cathode and anode chambers is 6:1:9. The membrane used is a cation exchange membrane, and the cathode and anode are Pb / PbO respectively. 2 plate and stainless st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com