Cell-free and minimized metabolic reaction cascades for the production of chemicals
A chemical, cell-free enzyme system technology, applied in the field of preparation of target chemicals, can solve the problems of expensive methods, unstable technology, low efficiency, etc., and achieve the effect of high cost efficiency and complexity elimination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0140] According to a preferred aspect, the preparation method of the present invention includes the following 4 steps:
[0141] Step I: Use microbial cells to prepare enzymes ("target enzymes") for the conversion of carbon sources into chemicals (also referred to herein as "target chemicals" or "target organic compounds");
[0142] Step II: Release the target enzyme from the microbial cell used in Step I, preferably combined with the release of cofactors and the inactivation of other non-target enzyme activities; or purify the target enzyme from the non-target enzyme activity, preferably with the cofactor Release combined
[0143] Step III: Contact the target enzyme of Step II with the carbon source under conditions suitable for the conversion of the carbon source to the target chemical;
[0144] Step IV: Separate the target chemical from the reaction mixture.
[0145] Enzyme selection and preparation:
[0146] In step I, the target enzyme is prepared using microbial cells. In one emb...
Embodiment 1
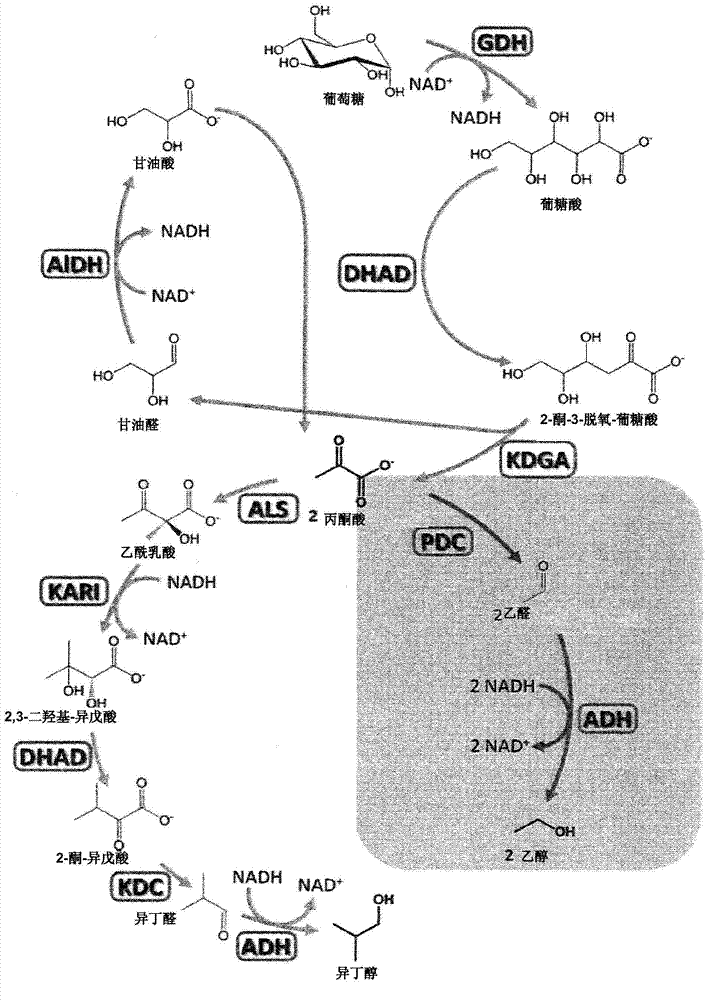
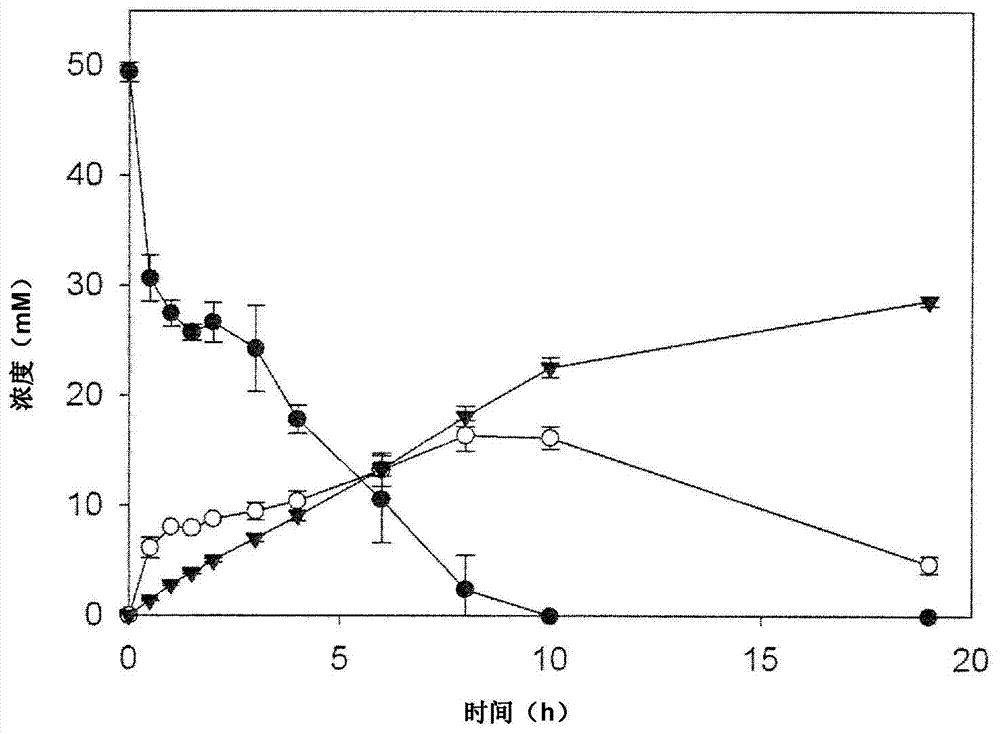
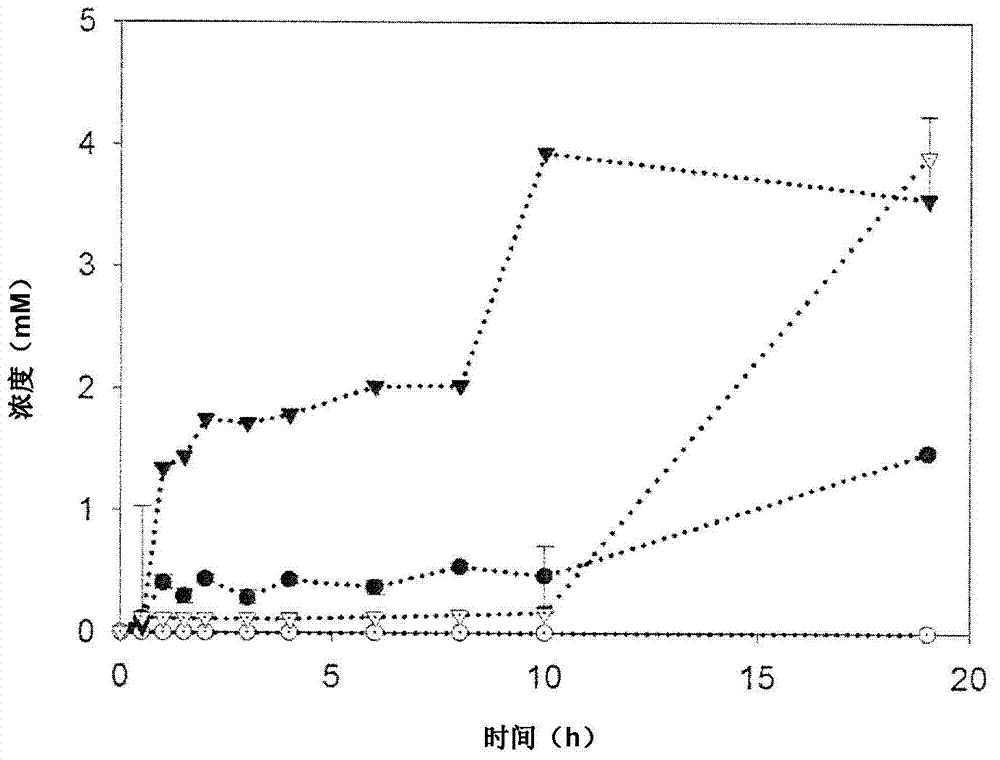
[0308] Example 1 (ethanol synthesis)
[0309] A general example of the flexibility of the cell-free synthesis toolbox is the use of an enzyme cascade that converts glucose or galactose to pyruvate with four enzymes to convert glucose or galactose to pyruvate. The four enzymes include glucose dehydrogenation. Enzymes (GDH), gluconic acid / glycerate / dihydroxy acid dehydratase (DHAD), 2-keto-3-deoxygluconic acid aldolase (KDGA) and glyceraldehyde dehydrogenase (ALDH). The ALDH used in this example is defined by SEQ ID NO 10 constructed in Example 4.
[0310] In the next two-step reaction, pyruvate passes through pyruvate decarboxylase (PDC) (J. Mol. Catal. B-Enzym. 2009, 61, 30-35) and alcohol dehydrogenase (ADH) (Protein Eng. 1998, 11, 925-930) and converted into acetaldehyde and then into ethanol. Due to its relatively high thermal tolerance and activity, PDC derived from Zymomonas mobilis was chosen. Although it is a mesophilic source, Zymomonas mobilis PDC is heat stable up to ...
Embodiment 2
[0315] Example 2 (Isobutanol synthesis)
[0316] This example shows the successful conversion of pyruvate to isobutanol using only four additional enzymes (see Figure 2 and Table 2) in a completely cell-free environment. Initially, two pyruvate molecules are combined by acetolactate synthase (ALS) (FEMS Microbiol. Lett. 2007, 272, 30-34) to obtain acetolactate, which is further passed by ketol acid reductoisomerase (KARI) ( Accounts Chem. Res. 2001, 34, 399-408) to obtain natural DHAD substrate dihydroxyisovaleric acid. After DHAD, dihydroxyisovaleric acid is converted to 2-ketoisovaleric acid.
[0317] Table 2:
[0318]
[0319] Table 2: Enzymes used in the cell-free synthesis of isobutanol. a: For the activity of natural substrates, DHAD (respectively) for gluconic acid, glyceric acid and dihydroxyisovaleric acid as substrates, and ADH for acetaldehyde and isobutyraldehyde as substrates; b: higher than solubility, c: Enzyme is engineered; E50: ethanol concentration that causes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com