Preparation method for ceftaroline fosamil
A technology of ceftaroline axetil and cephalosporin, which is applied in the field of preparation of ceftaroline axetil, can solve the problems of unfavorable industrial production, unavailable raw materials, and many by-products, and achieve the effects of low cost, improved purity, and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
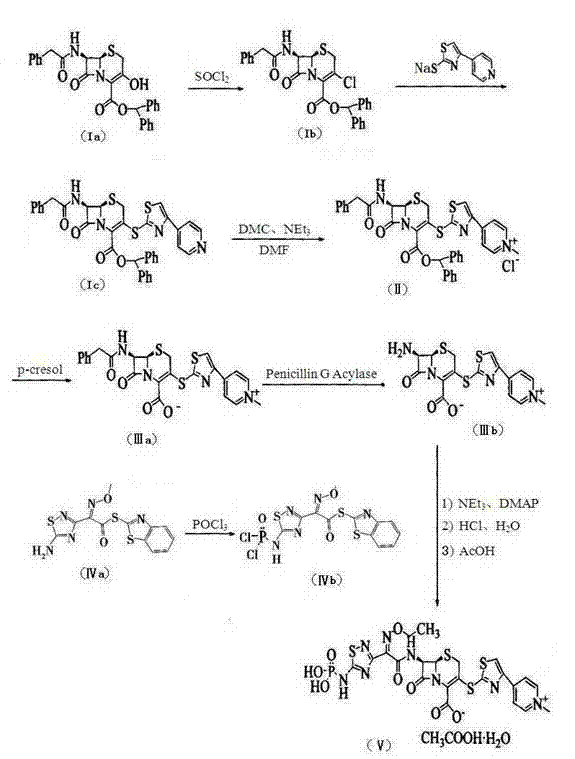
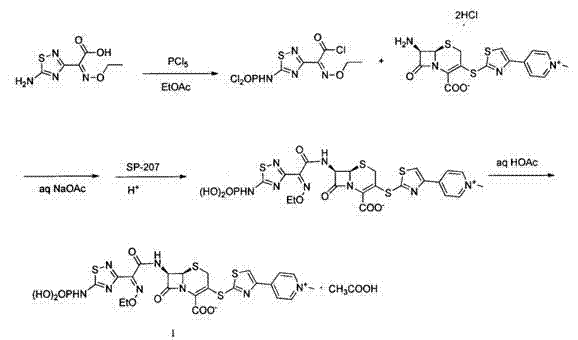
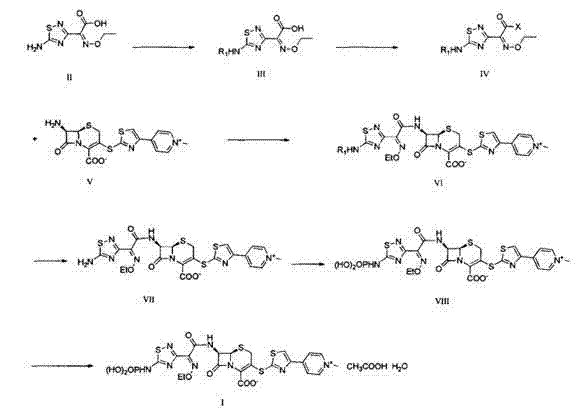
[0037] 1) 7β-[(phenylacetyl)amino]-3-[4-pyridyl-2-thiazolylthio]-3-cephem-4-carboxylic acid benzhydryl ester (formula IC ) preparation
[0038] At a temperature of 20-25°C, add 2000mL of dehydrated dimethylformamide into a dry reaction vessel, add 80g of thionyl chloride at room temperature, control the temperature at 30°C, stir for 1 hour, and then add 222g of 7-phenylacetyl Amino-3-hydroxy-3-cephalosporin-4-carboxylic acid benzhydryl ester (formula Ia, 0.4mol, industrial grade, content ≥ 90%), control the temperature at 25°C, stir for 8 hours, add 15 liters of ice water, filter the separated solid, rinse with a small amount of water, and suction filter to obtain about 228g of 7-phenylacetyl Amino-3-chloro-3-cephalosporin-4-carboxylic acid diphenylmethyl ester filter cake (formula Ib , the measured moisture is 15.8%, the equivalent dry weight is 192g, 0.36mol), and the molar yield is 90%.
[0039] 221.5g 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxylic acid diph...
Embodiment 2
[0050] 1) 7β-[(phenylacetyl)amino]-3-[4-pyridyl-2-thiazolylthio]-3-cephem-4-carboxylic acid benzhydryl ester (formula IC ) preparation
[0051] At a temperature of 20-25°C, add 2800mL of dehydrated dimethylformamide into a dry reaction vessel, add 120g of thionyl chloride at room temperature, control the temperature at 30°C, stir for 1 hour, and then add 278g of 7-phenylacetyl Amino-3-hydroxy-3-cephalosporin-4-carboxylic acid benzhydryl ester (formula Ia , 0.5mol, industrial grade, content ≥ 90%), control the temperature at 25°C, stir for 8 hours, add 15 liters of ice water, filter the separated solid, rinse with a small amount of water, and suction filter to obtain about 295g of 7-benzene Acetylamino-3-chloro-3-cephalosporin-4-carboxylic acid benzhydryl ester filter cake (formula Ib , the measured moisture is 18.0%, the equivalent dry weight is 242g, 0.455mol, and the molar yield is 91%).
[0052] The above-mentioned 7-phenylacetamido-3-chloro-3-cephalosporin-4-carboxyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com