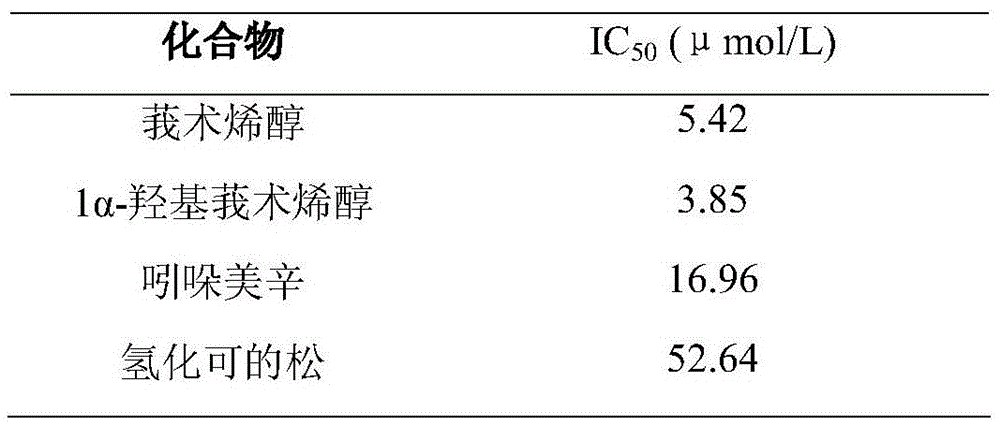
A preparation method and application of a microbial conversion product of curcumenol
A technology of microbial transformation and curcumenol, applied in the field of medicine, can solve problems affecting the formation of preparations and drug development, poor water solubility, difficulty in structural modification, etc., and achieve good research and development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: a) preparation of potato liquid culture medium: get fresh potato, peel, cut into 2cm 3 For small pieces, add 5 times the amount of water to boil, keep boiling for 30 minutes, filter the residue with gauze, add water to the filtrate (1 liter of culture medium per 200 grams of potatoes), and add 2% glucose after constant volume. Divide the prepared culture solution, 250mL Erlenmeyer flask can be filled with 60mL culture solution, and 500mL Erlenmeyer flask can be filled with 200mL culture solution. Seal the mouth of the bottle with gauze and kraft paper, and put it in an autoclave for sterilization. The sterilization condition is 115°C for 30 minutes.
[0019] b) Microbial culture: Inoculate Mucorspinosus AS3.2450 from Potato Dextrose Agar (PDA) (inoculation area is about 0.05cm 2 ) into the potato liquid medium prepared above, placed in a shaker at 160 rpm, and cultured at 26°C for 2 days.
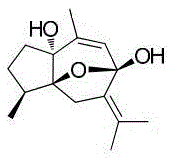
[0020] c) Adding the substrate: dissolve the substrate curcumen...
Embodiment 2
[0023] Example 2: a) Preparation of potato liquid medium: same as Example 1.
[0024] b) Microbial culture: Inoculate Mucorspinosus AS3.2450 from Potato Dextrose Agar (PDA) (inoculation area is about 0.05cm 2 ) into the potato liquid medium prepared above, placed in a shaker at 160 rpm, and cultured at 26°C for 2 days.
[0025] c) Adding the substrate: dissolve the substrate curcumenol with an appropriate amount of acetone to prepare a 10 mg / mL acetone solution. A total of 200 mg of curcumenol was added to the microbial fermentation broth in step b) that had been cultured for 2 days, and the culture was continued for 8 days under the same conditions (160 rpm, 26° C.).
[0026] d) Treatment of fermented liquid: Same as in Example 1, 755 mg of crude extract was obtained.
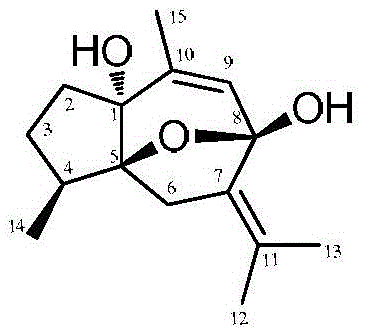
[0027] e) The silica gel column chromatographic separation method was the same as in Example 1, and the target conversion product 1α-hydroxycurcumenol (100.8 mg) was obtained with a yield of 50.4%.
Embodiment 3
[0028] Example 3: a) Preparation of potato liquid medium: same as Example 1.
[0029] b) Microbial culture: Inoculate Mucorspinosus AS3.2450 from Potato Dextrose Agar (PDA) (inoculation area is about 0.05cm 2 ) into the potato liquid medium prepared above, placed in a shaker at 160 rpm, and cultured at 26°C for 3 days.
[0030] c) Adding the substrate: dissolve the substrate curcumenol with an appropriate amount of acetone to prepare a 10 mg / mL acetone solution. A total of 200 mg of curcumenol was added to the microbial fermentation broth of step b) that had been cultured for 3 days, and the culture was continued for 4 days under the same conditions (160 rpm, 26° C.).
[0031] d) Treatment of fermented liquid: Same as in Example 1, 630 mg of crude extract was obtained.
[0032] e) The silica gel column chromatographic separation method was the same as in Example 1, and the target conversion product 1α-hydroxycurcumenol (79.2 mg) was obtained with a yield of 39.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com