Thioether monooxygenase PMO-3546 and application thereof
A thioether monooxygenase and protein technology, which is applied in the fields of biocatalysis, biosynthesis and molecular biology, can solve the shortage of high enantioselective thioether monooxygenase, which cannot meet the needs of green industrial production and low catalytic efficiency And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Acquisition of gene encoding thioether monooxygenase
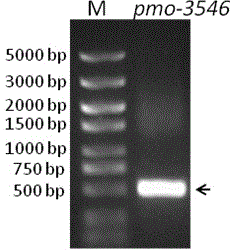
[0041] By analyzing the genome sequence of Pseudomonas strain, a monooxygenase sequence with a length of 486 bp was determined, a pair of specific primers were designed and amplified by PCR technology, and the full-length coding frame sequence of the gene was finally obtained. The specific method is as follows: using the extracted genomic DNA as a template, use primers F: 5'- CTGCGGATCCATGATCGACGCAACCGTCT -3' and R: 5'- AACCAAGCTTGGCA
[0042]AAGGCCGGCAG-3' was PCR amplified. The PCR reaction system is as follows: 12.5 ul of 2×TaqPCR Master Mix, 1 ul of genomic DNA, 0.5 ul of upstream and downstream primers, and 10.5 ul of ddH2O. PCR reaction conditions: 95°C for 10 min; cycle of 98°C for 10 s, 57°C for 30 s, 72°C for 90 s; extension at 72°C for 10 min, 30 cycles. The PCR product was electrophoresed on a 1% agarose gel to verify whether a fragment conforming to the size of the target gene was obtaine...
Embodiment 2
[0043] Example 2. Construction of thioether monooxygenase gene expression vector
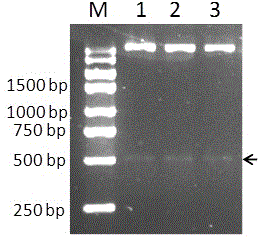
[0044] Using the pET32a(+) vector (purchased from Novagen) as the backbone, the monooxygenase gene was constructed pmo-3546 prokaryotic expression vector. The specific method is as follows: Use the DNA gel recovery kit to recover pmo-3546 PCR products, with restriction enzymes Bam h and Hin d The PCR product and the vector pET32a(+) were double digested, and the recovered target fragment and vector were digested. After ligation with T4 DNA ligase at 16 °C for 4 h, the ligation product was transformed into Escherichia coli DH5α. After culturing overnight, pick out the grown monoclonal colony and shake the bacteria and extract the plasmid, use Bam h and Hin d Double-enzyme digestion detection of recombinant plasmids. Select the positive recombinant plasmid pET32a- pmo-3546 DNA sequencing is performed to ensure the sequence is accurate. Finally, the positive recombinant ...
Embodiment 3
[0045] Example 3. Recombinant expression and detection of thioether monooxygenase protein
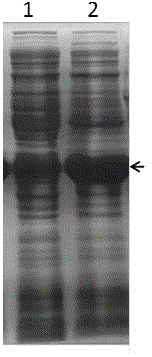
[0046] Will be stored in glycerol containing pET32a- pmo-3546 After activation of the genetically engineered bacteria BL21(DE3) with the plasmid, pick a single colony in 3 ml of liquid LB medium containing the corresponding antibiotics, culture with shaking at 37 °C for 12 h, and transfer to a fresh medium containing 1% of the inoculum the next day. In 50 mL LB liquid medium of antibiotics, shake culture at 37°C 250 rpm with OD600 of 0.6 (about 3h), add IPTG with a final concentration of 0.5 mM, and induce culture at 25°C 160 rpm for 8h. After induction, collect the cells by centrifugation at 8,000 rpm / min for 5 min, resuspend the cells in PBS buffer, break up the cells by ultrasonic, and centrifuge at 15,000 rpm for 5 min to remove cell debris, take the supernatant and mix with 5x loading buffer, and place in SDS-PAGE electrophoresis analysis was performed after heating in a consta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com