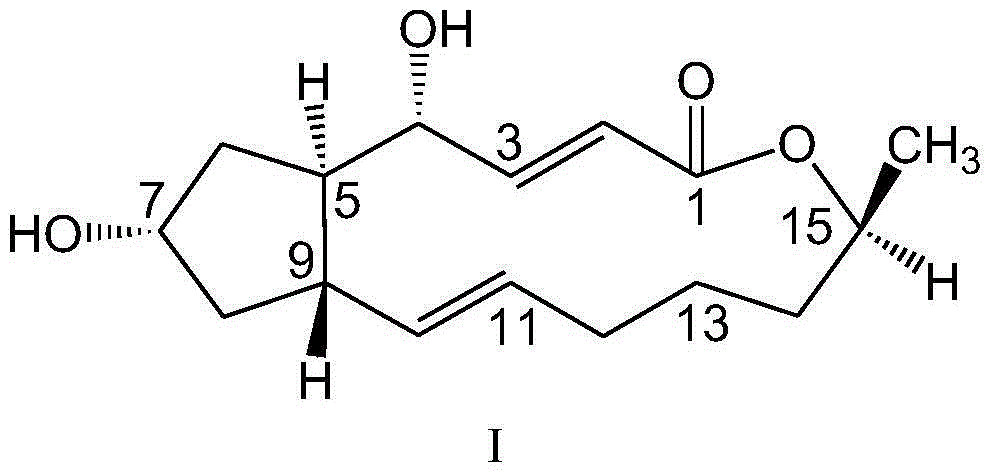
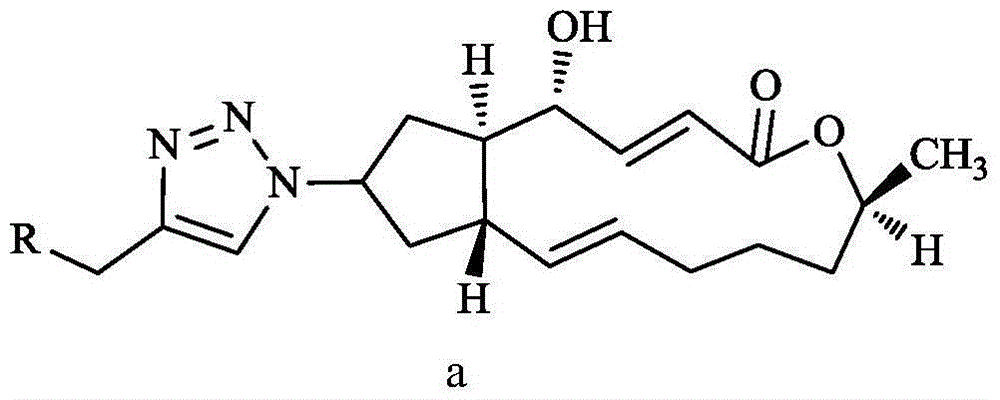
Preparation and anti-tumor application of 7-N3-brefeldin A and 1,2,3-triazole derivative thereof
A brefeldin and 7-N3- technology is applied in the fields of preparation and anti-tumor application of 7-N3-brefeldin A and its 1,2,3-triazole derivatives, and can Solve the problems of unsatisfactory, short plasma half-life, poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of 7-O-methylsulfonyl-BFA (formula IV)
[0069]
[0070] Under nitrogen protection, add 6 mL of pyridine to a 50 mL round bottom flask with a magnetic stirrer, then add BFA (500 mg, 1.79 mmol), then add triethylamine (240 mg, 2.38 mmol) and start stirring at -20 ° C; Methanesulfonyl chloride (273 mg, 2.38 mmol) was added dropwise to the mixture (dropwise for 10 min), and after the drop was completed, the mixture was reacted at -20°C for 2 h to terminate the reaction. After the reaction solution was concentrated, 30ml of ethyl acetate was added for dilution, washed with 5% citric acid solution (2×10ml), saturated sodium bicarbonate solution (2×10ml), saturated sodium chloride solution (2×10ml), and organic phase, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition that the developer ethyl acetate (E):petroleum ether...
Embodiment 2
[0073] Example 2: 7-N 3 - Preparation of BFA (Formula II)
[0074]
[0075] Under the protection of helium, 10 mL of N, N-dimethylformamide was added to a 50 mL round bottom flask with a magnetic stirrer, and then compound IV 7-O-methylsulfonyl-BFA (400 mg, 1.12 mmol) was added, Then sodium azide (217mg, 3.35mmol) was added and stirred at reflux at 70°C for 4h to terminate the reaction. After the reaction solution was cooled and concentrated, 30ml of ethyl acetate was added to dilute, washed with 5% citric acid solution (2×10ml), saturated sodium bicarbonate solution (2×10ml), saturated sodium chloride solution (2×10ml), and combined The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product. The crude product was separated by thin-layer chromatography under the condition of developing solvent E / P volume ratio = 1:5 to obtain compound II 7-N 3 - BFA (Rf = 0.3, yield 83.40%).
[0076] Compound Character...
Embodiment 3
[0084] Embodiment 3: the preparation of compound a-1
[0085]
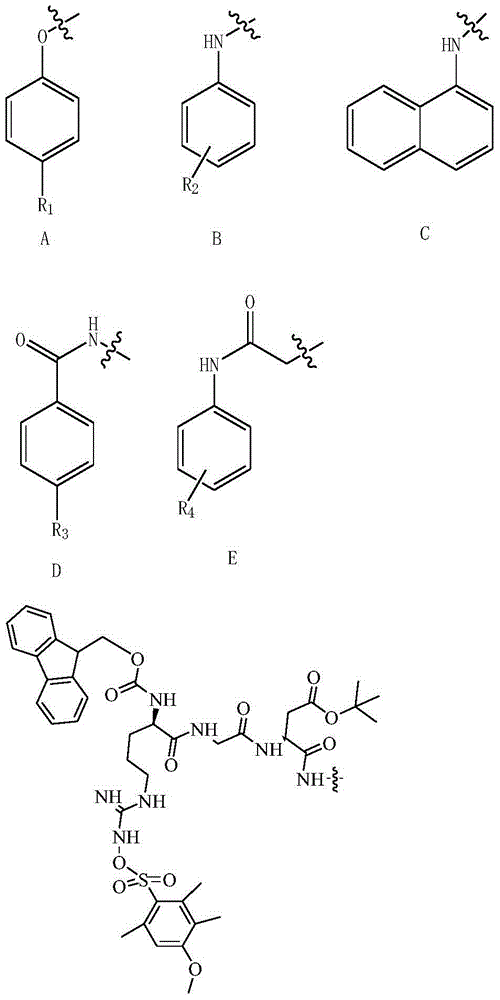
[0086] Add THF:H to a 50 mL round bottom flask with a magnetic stir bar 2 O volume ratio=1:1 solvent (10ml), then add 30.5mg 7-N 3 - BFA (0.1 mmol), CuSO 4 .5H 2 O (0.01mmol, 2.5mg) and ascorbic acid (Vc0.015mmol, 2.64mg), start stirring; after stirring and dissolving, add alkyne III-A1 (R 1 =H, 0.15mmol, 19.8mg), stirred and reacted at room temperature for 6h, and the reaction process was detected and tracked by TLC. After the reaction was completed and cooled, the solvent was concentrated, and CH 2 Cl 2 Extract (3x20ml), combine the organic phases and wash with water, then add anhydrous MgSO 4 After drying, filtering and concentrating the filtrate, the obtained crude product was separated and purified by thin layer chromatography (E:P volume ratio=5:1) to obtain the corresponding product with a yield of 65.4%.
[0087] Compound Characterization:
[0088] Compound (a-1): Pale yellow solid. Yield 65.5%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com