Method for intrathecally injecting nerve regeneration promoting injection
A technique of nerve regeneration and intrathecal injection, applied in the field of medicine, can solve the problems such as effective therapeutic concentration and large molecular weight in parts without obvious and incapable spinal cord injury, and achieves reduction of secondary injury, good biological safety, and avoidance of molecular weight. big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 acute toxicity test
[0018] 40 healthy adult Wistar rats (SPF grade, provided by the Experimental Animal Center of the Academy of Military Medical Sciences, license number: SCXK-jun-2007-004), male or female, weighing 210-270 g, were randomly divided into 5 groups (L1 ~L5), 8 rats in each group, after successful anesthesia by intraperitoneal injection of 1% pentobarbital sodium (40mg / kg), the N6 compound was prepared immediately, respectively according to the first group 41.5μL / kg, the second group 83μL / kg, Inject 166 μL / kg in the third group, 332 μL / kg in the fourth group, and 664 μL / kg in the fifth group into the subarachnoid space, tail vein, muscle, and abdominal cavity of rats at L2 and 3 levels, 2 rats for each part, and perform acute treatment. For the toxicity test, observe for 3 days, and record whether the experimental animals have adverse reactions and death.
[0019] Administration method: (1) subarachnoid route: after successful anesthesia, t...
Embodiment 2
[0021] Embodiment 2 subacute toxicity test
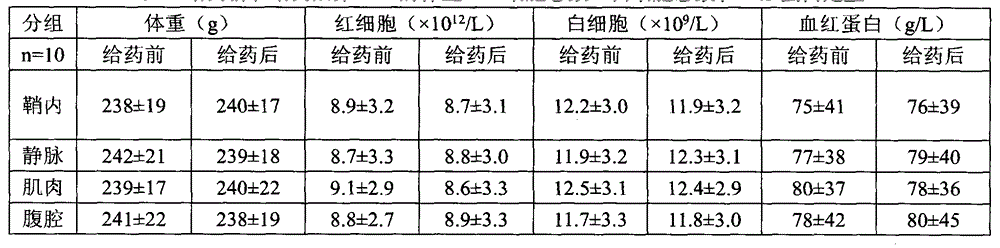
[0022] 40 healthy adult Wistar rats (the same source as in Example 1), male or female, with a body weight of 210-270 g, were randomly divided into 4 groups (L1-L4) according to the injection site, 10 in each group, and measured body weight, white blood cell count, Red blood cell count and hemoglobin content, after successful intraperitoneal injection of 1% pentobarbital sodium (40mg / kg), the N6 compound was prepared immediately and injected into the subarachnoid of rats at L2 and 3 levels respectively at a therapeutic dose of 166 μL / kg Cavity, tail vein, muscle, abdominal cavity, the administration method is the same as that of Example 1. After 2 weeks, body weight, white blood cell count, red blood cell count and hemoglobin content were measured and statistically processed.
[0023] Experimental animals were treated in two ways: 5 animals were randomly selected from each group and killed, and the liver, spleen, heart and kidney wer...
Embodiment 3
[0027] Example 3 In Vitro Hemolysis Test
[0028] Take 7 test tubes and prepare N6 complex immediately. Inject 41.5 μL, 83 μL, 166 μL, 332 μL, and 664 μL of N6 complex into test tubes 1 to 5 respectively, add human erythrocyte suspension and normal saline in equal amounts, and use test tube 6 as a blank control. Test tube No. 7 was used as a complete hemolysis control, placed in a 37°C incubator, and observed for hemolysis at 1 hour, 2 hours, and 3 hours respectively.
[0029] After 3 hours of observation, there was no hemolysis in test tubes 1 to 5. The upper layer was a light pink clear liquid, and the lower layer was red blood cell deposits. No red blood cell aggregation or rupture was found in the smear microscope.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com