A kind of ammonia nitrogen detection test paper and application method thereof
A technology for detecting test paper and ammonia nitrogen, applied in the field of chemical analysis, can solve the problems of limiting the rapid determination of ammonia nitrogen, cumbersome measurement steps, long color development time, etc., and achieves the effects of short color development time, low price and strong mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Prepare the ammonia-free aqueous solution containing sodium salicylate 280g / L, potassium sodium tartrate 50g / L and sodium nitroferricyanide 0.40g / L. Immerse all the quantitative medium-speed filter paper in the solution, and shake it on a constant temperature oscillator for 90 minutes. Take out the filter paper, place it in parallel at room temperature, hang it in the air to dry, cut it and seal it for later use, and then make ammonia nitrogen test paper.
[0021] 2. Activator: take the calibrated analytically pure sodium hypochlorite, dilute it with ammonia-free water and sodium hydroxide solution (2mol / L) to form sodium hypochlorite containing an available chlorine concentration of 3.5g / L and a free alkali concentration of 0.75mol / L (calculated as NaOH) Use liquid.
[0022] 3. Preparation of standard color chart: Accurately weigh ammonium chloride (dried at 110°C for 2 hours) and dissolve it in ammonia-free water to prepare nitrogen-containing standard solutions w...
Embodiment 2
[0027] 1. Prepare the ammonia-free aqueous solution that concentration is to contain sodium salicylate 380g / L, potassium sodium tartrate 68g / L and sodium nitroferricyanide concentration 0.54g / L. Immerse all the quantitative medium-speed filter paper in the solution, and shake it on a constant temperature oscillator for 90 minutes. Take out the filter paper, place it in parallel at room temperature, hang it in the air to dry, cut it and seal it for later use, and then make ammonia nitrogen test paper.
[0028] 2. Activator: take the calibrated analytically pure sodium hypochlorite, dilute it with ammonia-free water and sodium hydroxide solution (2mol / L) to form sodium hypochlorite containing an available chlorine concentration of 3.5g / L and a free alkali concentration of 0.75mol / L (calculated as NaOH) Use liquid.
[0029] 3. Preparation of standard color chart: Accurately weigh ammonium chloride (dried at 110°C for 2 hours) and dissolve it in ammonia-free water to prepare nitr...
Embodiment 3
[0032] Other steps are the same as in Example 1, except that the ammonia-free aqueous solution contains 330 g / L of sodium salicylate, 59 g / L of potassium sodium tartrate and 0.47 g / L of sodium nitroferricyanide.
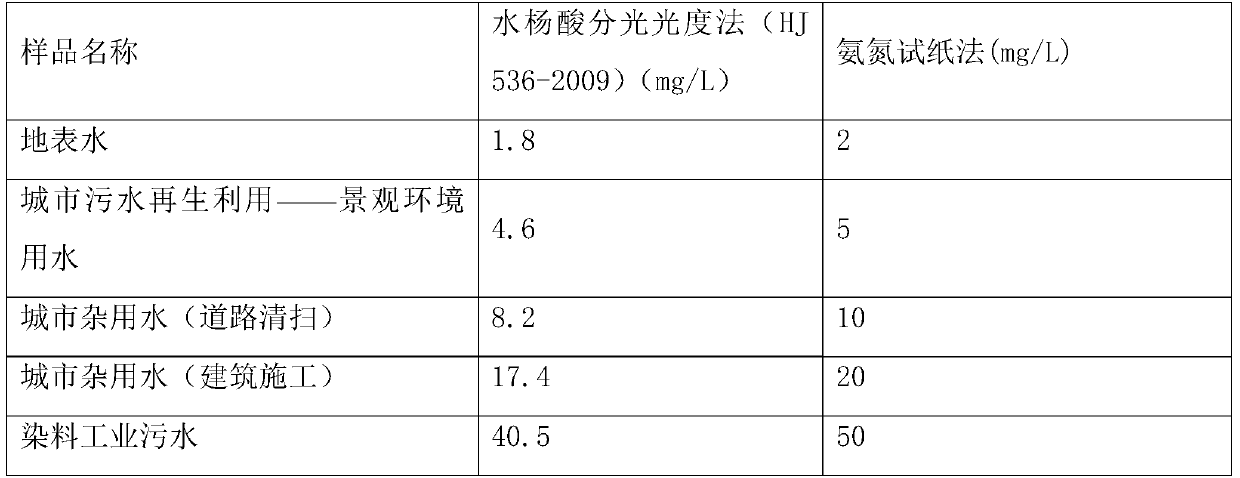
[0033] The result measured with this test paper is compared with the result measured by the standard method with Table 1 in Example 1, consistent with the standard result, and the test paper can accurately measure the ammonia nitrogen content in various water qualities.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com