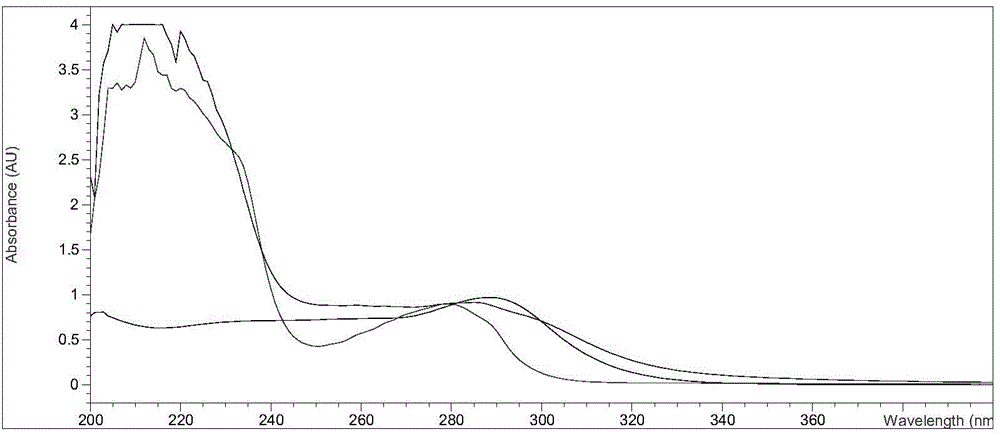
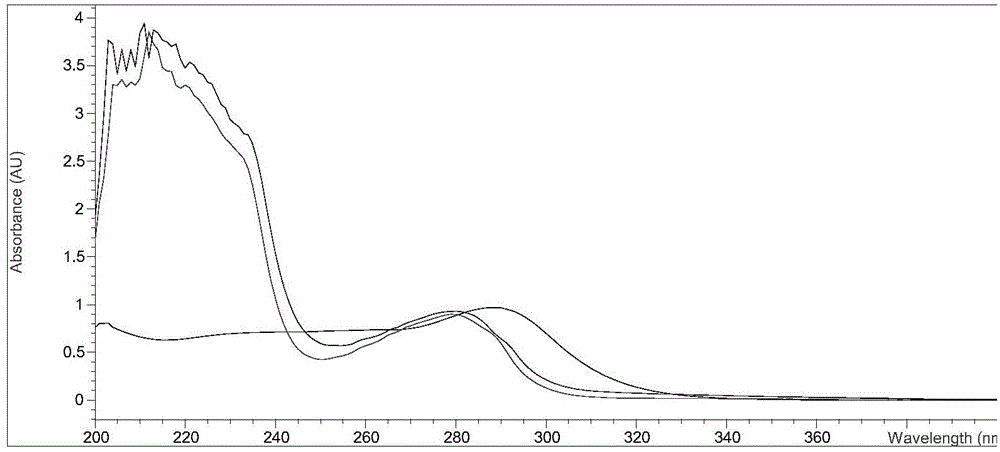
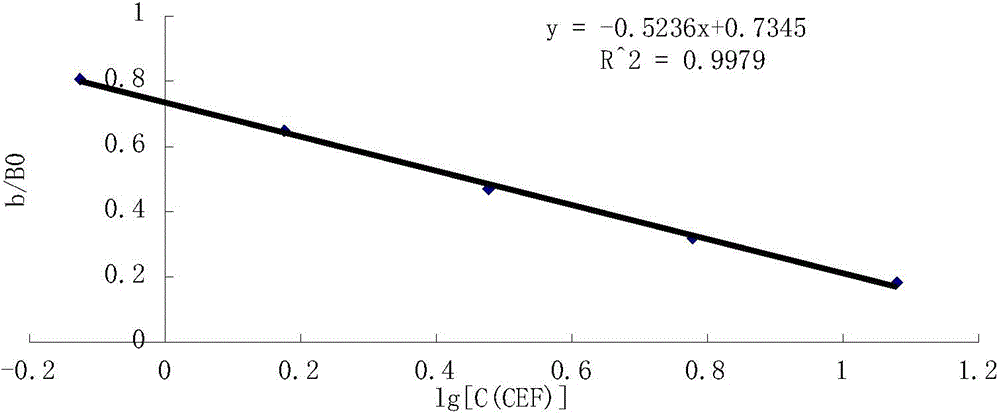
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cephalosporin antibiotics
A monoclonal antibody and cephalosporin technology, which is applied in the field of drug residue analysis and immunology, can solve the problems of insufficient drug types and failure to identify cephalosporin antibiotics, etc., and achieve simple sample processing methods, high accuracy, and time saving and cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 immunogen and coating former
[0032] 1.1 Preparation of immunogen
[0033] Dissolve 50 mg of ceftiofur in 1 mL of dimethylformamide (DMF) to obtain liquid A. Weigh 66mg of HSA and dissolve it in 10mL of PBS to make solution B. Add 20mg of N,N'-dicyclohexylcarbodiimide (DCC) and 10mg of N-hydroxysuccinimide (NHS) to solution A respectively, and react at room temperature for 24 hours. After the reaction is completed, centrifuge to remove the precipitate. Drop into solution B, and react in ice bath for 24h. After the reaction is completed, transfer the reaction solution into a dialysis bag, dialyze in PBS at 4°C for 3 days, replace the dialysate every 4-6 hours, and freeze-dry the sample after the dialysis to obtain the conjugate ceftiofur-HSA. Store at 20°C.
[0034] 1.2 Preparation of coating agent
[0035] Dissolve 120 mg of OVA in 8 mL of carbonate buffered saline (CBS) to form A liquid. Ceftiofur 50mg is dissolved in 2mL CBS, whi...
Embodiment 2
[0036] The preparation of embodiment 2 monoclonal antibody
[0037] Preparation of hybridoma cells: referring to "Animal Immunology" by Yang Hanchun, Balb / C mice (purchased from the Experimental Animal Center of Hubei Academy of Medical Sciences) were immunized with the immunogen ceftiofur-HSA conjugate prepared in Example 1. The immunization procedure is as follows: for basic immunization, a protein emulsion containing 100 μg of immunogen emulsified with an equal volume of Freund’s complete adjuvant is injected subcutaneously at the back of the neck of the mouse; Protein emulsion of immunogen for booster immunization. From the third immunization, the tail blood was collected on the 8th day after each immunization, the serum was separated, and the serum antibody titer was detected by indirect ELISA method. Immunization qualified mice (high titer, good sensitivity) stop immunization in preparation for fusion. At the time of fusion, take a Balb / C mouse that has undergone the f...
Embodiment 3
[0042] The establishment of embodiment 3 indirect competition ELISA detection method
[0043] 3.1 Preparation of reagents (the reagents used in this example were prepared by the following methods unless otherwise specified)
[0044] Phosphate buffer: NaCl 8.0g, KH 2 PO 4 0.2g, Na 2 HPO 4 12H 2 O 2.9g, KCl 0.2g, add double distilled water to 1000mL, adjust pH to 7.4;
[0045] Coating solution: Take Na 2 CO 3 1.59g, NaHCO 3 2.93g, add triple distilled water to 1000mL, adjust the pH value to 9.6;
[0046] Washing liquid: NaCl 8.0g, KH 2 PO 4 0.2g, Na 2 HPO 4 12H 2 O 2.9g, KCl 0.2g, Tween 200.5mL, add double distilled water to 1000mL, adjust pH to 7.4;
[0047] Blocking solution: Ovalbumin 1g dissolved in 100mL phosphate buffer;
[0048] Substrate solution A: 3,3',5',5-tetramethylbenzidinediamine (TMB) 200mg, absolute ethanol 100mL, add double distilled water to 1000mL;
[0049] Substrate B: Na 2 HPO 4 14.6g, citric acid 9.3g, 0.75% urea hydrogen peroxide 6.4mL, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com