Prednisone pulse release tablet
A pulsed release, prednisone technology, applied in the directions of organic active ingredients, non-central analgesics, pharmaceutical formulations, etc., to achieve the effect of convenient and feasible medication, symptom relief, and easy large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
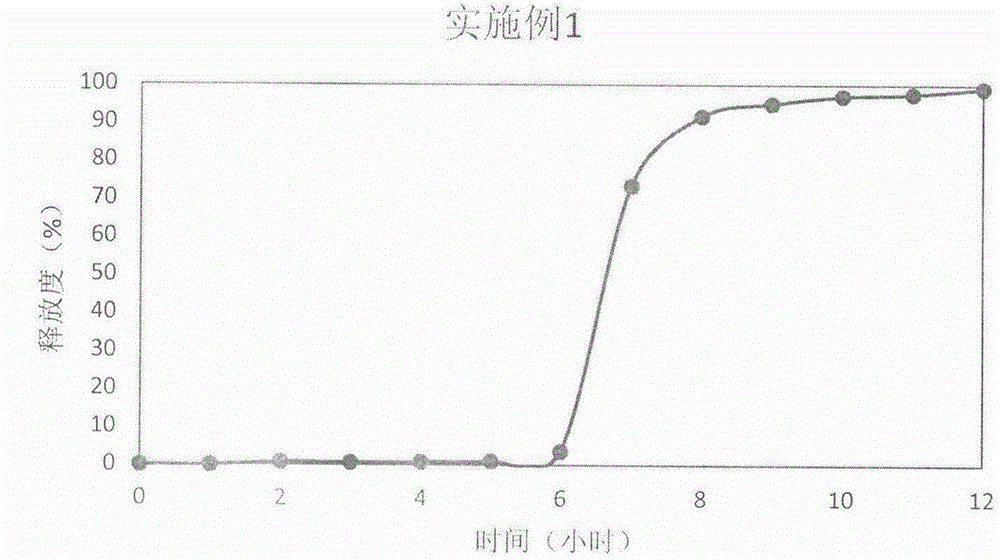
Embodiment 1
[0033] Prednisone
[0034]Preparation process: After the above-mentioned prednisone and auxiliary materials are fully mixed, the powder is directly compressed into tablets to obtain immediate-release tablet cores.
[0035] Controlled Release Coating Formulation
[0036] HPMC E50 (Colorcon)
[0037] Preparation process: Fill 150mg of HPMC E50 cup body material into a 9mm shallow concave die, press lightly to form a cup body layer; then, place the immediate-release tablet core in the center of the cup body layer, press lightly to make the tablet core and cup body part Flat; finally add 80mg of top layer material (30% mannitol 70% HPMC E50), and carry out direct powder compression to get final product.
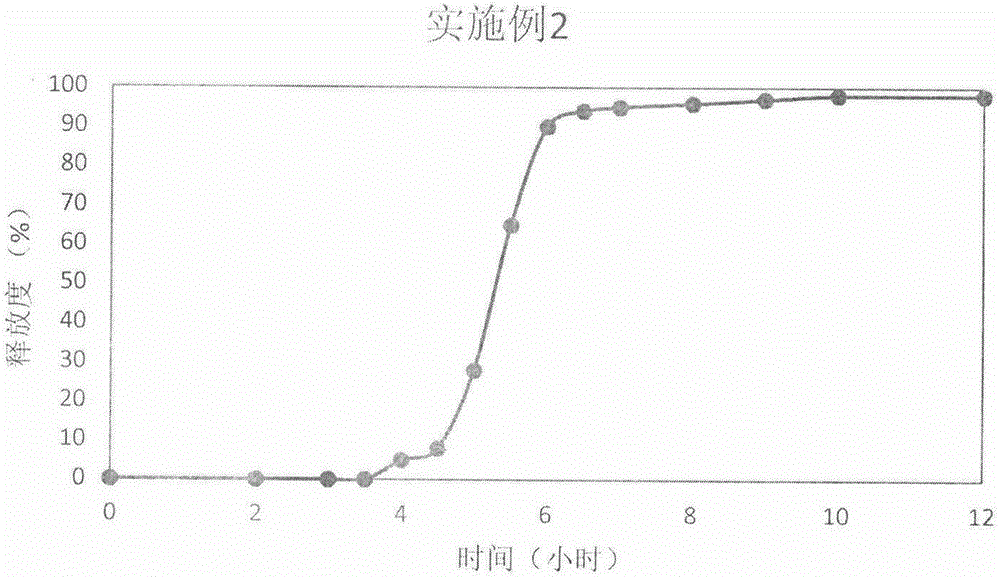
Embodiment 2
[0039] Prednisone
[0040] Preparation process: After the above-mentioned prednisone and auxiliary materials are fully mixed, the powder is directly compressed into tablets to obtain immediate-release tablet cores.
[0041] Controlled Release Coating Formulation
[0042] HPMC E50 (Colorcon)
[0043] Preparation process: 150mg of cup material (10% HPMC E6, 90% HPMC E50) is filled into a 9mm shallow concave die, and lightly pressed to form a cup layer; then, the immediate-release tablet core is placed in the center of the cup layer, Gently press to make the tablet core and the cup part flush; finally add 80 mg of top layer material (30% mannitol, 70% HPMC E50), and carry out direct powder compression to get final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com