Sulpiride tablet and preparation method thereof
A technology of sulpiride tablets and tablets, which is applied in the direction of pharmaceutical formulas, medical preparations of non-active ingredients, pill delivery, etc., can solve problems such as unsatisfactory dissolution rate of sulpiride tablets, and improve bioavailability and compressibility Good, saving consumption and cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) The preparation of solid dispersion carrier, PEG4000 300g, PVPk30 20g are joined in the 95% (ml / ml) ethanol of formula quantity 2 / 3, heat and stir until PEG4000 and PVPk30 dissolve completely, get adjuvant solution; 1000g sulpiride is dissolved in the 95% (ml / ml) ethanol of recipe quantity (remainder) 1 / 3 to obtain main drug solution; Described adjuvant solution and described main drug solution are mixed uniformly to obtain mixed solution; The solution was vacuum-dried for 12 hours (vacuum degree less than 10Pa), the drying temperature was 35°C, and the moisture content was controlled to be less than 1% (mass percentage);
[0018] (2) Tablet preparation, pulverize the material obtained in step (1), and pass through a 100-mesh standard sieve to obtain a solid dispersion powder; put the gained solid dispersion powder, 200 g of lactose, and 300 g of microcrystalline cellulose into a granulator, and dry mix 5min, add 40% (ml / ml) ethanol of 150g as binding agent, set the...
Embodiment 2
[0023] (1) The preparation of solid dispersion carrier, PEG4000 300g, PVPk30 30g are joined in the 95% (ml / ml) ethanol of formula quantity 2 / 3, heat and stir until PEG4000 and PVPk30 dissolve completely, get adjuvant solution; 1000g sulpiride is dissolved in the 95% (ml / ml) ethanol of recipe quantity (remainder) 1 / 3 to obtain main drug solution; Described adjuvant solution and described main drug solution are mixed uniformly to obtain mixed solution; The solution was vacuum-dried for 24 hours (vacuum degree less than 10Pa), the drying temperature was 55°C, and the moisture content was controlled to be less than 1% (mass percentage);
[0024] (2) Tablet preparation, pulverize the material obtained in step (1), and pass through a 120-mesh standard sieve to obtain a solid dispersion powder; put the gained solid dispersion powder, 200 g of lactose, and 300 g of microcrystalline cellulose into a granulator, and dry mix 10min, add the 45% (ml / ml) ethanol of 200g as binding agent, se...
Embodiment 3
[0026] Tablet Quality Check
[0027] 1. Appearance: The surface of the sulpiride tablet prepared in Examples 1 and 2 is smooth, and it is brighter and whiter than the tablet prepared by traditional techniques.
[0028] 2. Dissolution rate:
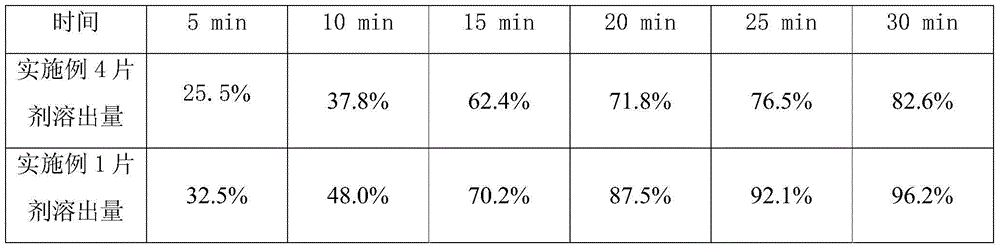
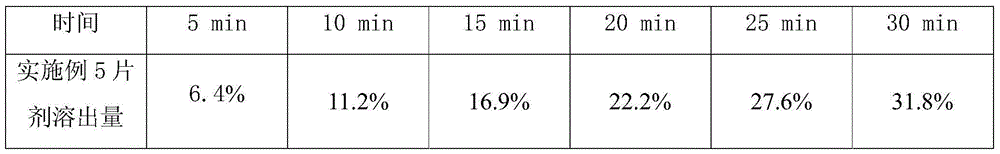
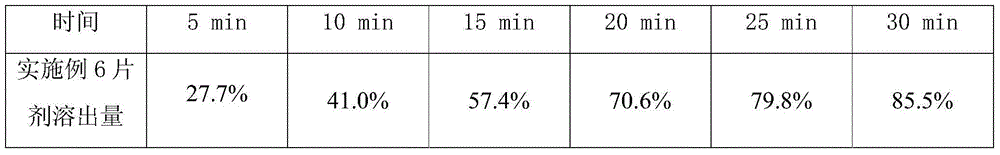
[0029] Embodiment 1, 2 gained sulpiride tablet respectively gets 6, checks dissolution rate according to Chinese Pharmacopoeia 2010 edition two appendix XC, the dissolution amount of the tablet prepared in embodiment 1, 2 is all more than 85% in 30 minutes (according to labeling meter).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com