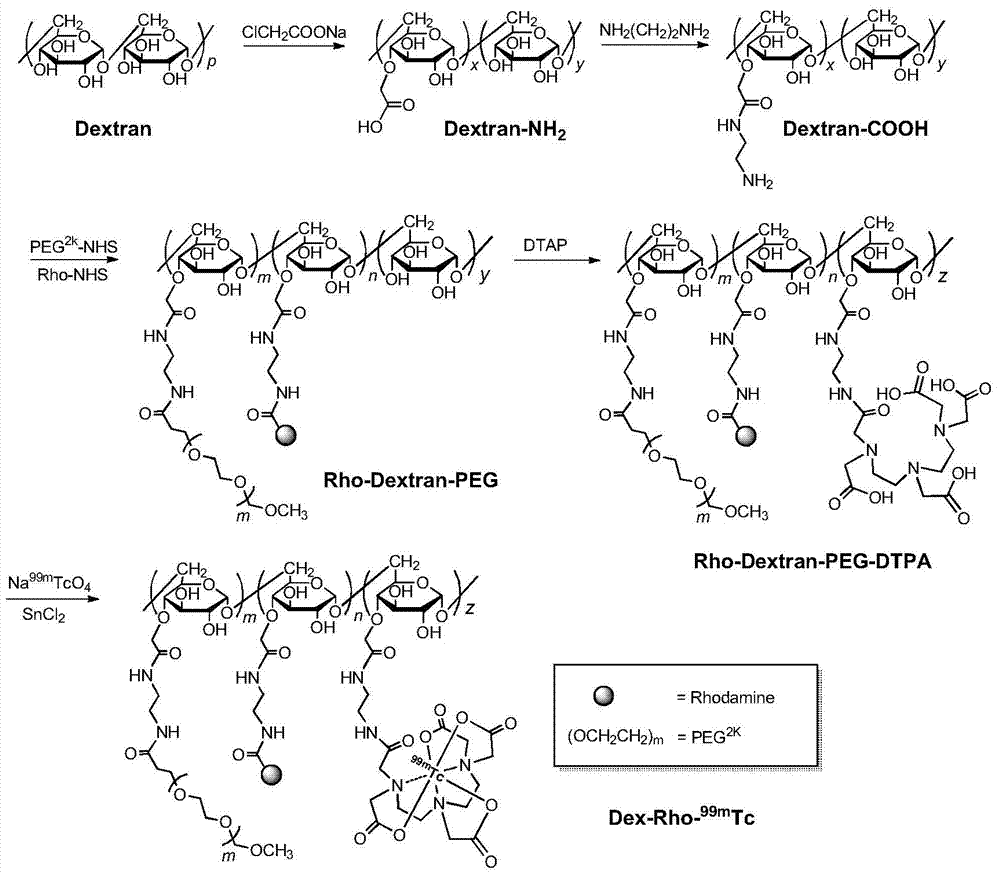
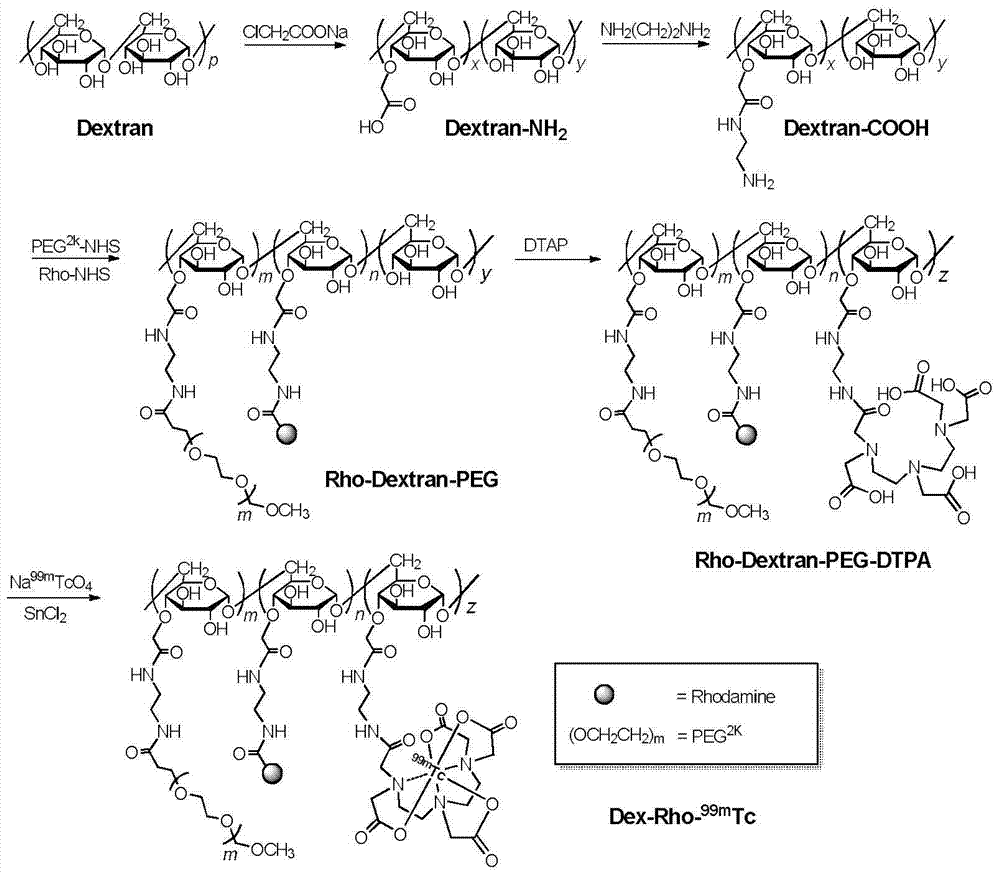
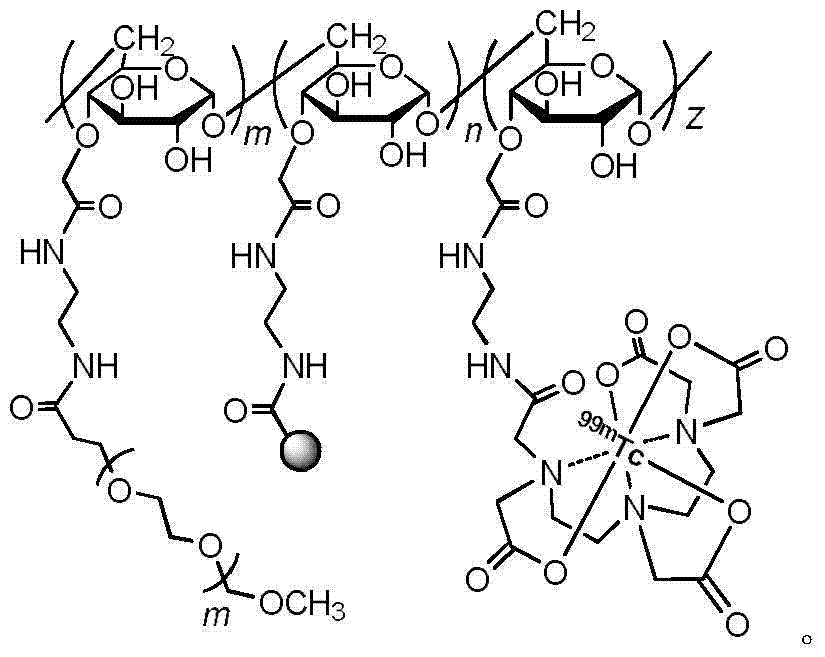
Dual-modal nano imaging drug Dex-Rho-99mTc based on glucan
A technology of -dex-rho-99mtc and rho-dex-peg-dtpa-99mtc is applied in the field of imaging medicine, imaging diagnosis, and the preparation of dual-mode nano-imaging medicine to improve biocompatibility and price The effect of low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Nano imaging drug carrier material Dex-NH 2 Synthesis
[0037] Dextran (200mg) with a molecular weight of 20kDa was dissolved in 7mL of 6M NaOH solution, cooled in an ice bath to 0°C, added sodium chloroacetate (503mg), reacted at 60°C for 50min, and then added dropwise to methanol to obtain a white flocculent precipitate Dex- COOH. Dex-COOH (200 mg) was dissolved in 20 ml of water (pH 3.0), and 1 ml of 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ, 320 mg) in DMF was added dropwise. This solution was dropped into 0.414mL ethylenediamine, and stirred at room temperature for 4h. The reaction solution was concentrated to 3 mL by rotary evaporation, and dropped into methanol to obtain the white floc product Dex-NH 2 .Dex-NH 2 .The aqueous solution was purified by ultrafiltration three times with an ultrafiltration tube with a molecular weight cut-off of 3kDa, and the concentrated solution was freeze-dried to obtain a white flocculent solid product, which is ...
Embodiment 2
[0041] (1) Synthesis of nanoimaging drug carrier material Dex-NH 2
[0042] Dextran (200mg) with a molecular weight of 20kDa was dissolved in 7mL of 6M NaOH solution, cooled in an ice bath to 0°C, added sodium chloroacetate (503mg), reacted at 60°C for 50min, and then added dropwise to methanol to obtain a white flocculent precipitate Dex- COOH. Dex-COOH (200 mg,) was dissolved in 20 ml of water (pH 3.0), and 1 ml of 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ, 320 mg) in DMF was added dropwise . This solution was dropped into 0.414mL ethylenediamine, and stirred at room temperature for 4h. The reaction solution was concentrated to 3 mL by rotary evaporation, and dropped into methanol to obtain the white floc product Dex-NH 2 .Dex-NH 2 .The aqueous solution was purified by ultrafiltration three times with an ultrafiltration tube with a molecular weight cut-off of 3kDa, and the concentrated solution was freeze-dried to obtain a white flocculent solid product, whic...
Embodiment 3
[0048] With the test method of Example 1, the molecular weight of the fixed carrier material Dextran is determined as 50kDa, and the molecular weight of PEG is changed to obtain the imaging drug Rho-Dex connected with 1kDa, 2kDa, 3.5kDa, 5kDa, 10kDa molecular weight PEG 50k -PEG-DTPA- 99m Tc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com