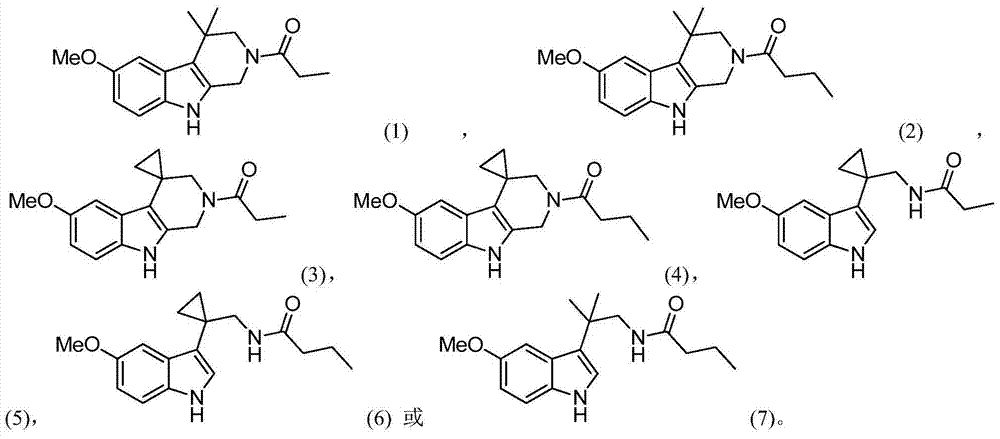
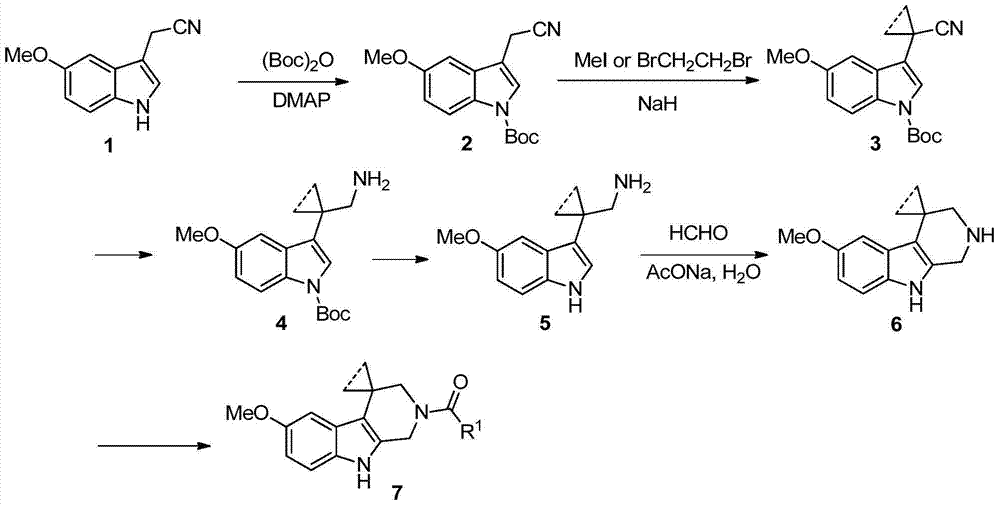
Indole derivatives and use thereof in medicine
A drug and pharmaceutical technology, applied in the treatment of central nervous system dysfunction, in the field of indole compounds, can solve problems such as shortening sleep latency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Example 1: 1-(6-methoxy-4,4-dimethyl-3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)propane Synthesis of yl-1-ones
[0189]
[0190] Step 1) Synthesis of tert-butyl-3-(cyanomethyl)-5-methoxyl-1H-indole-1-carboxylate
[0191] 2-(5-methoxy-1H-indol-3-yl)acetonitrile (5.59g, 30.0mmol), 4-dimethylaminopyridine (0.37g, 3.0mmol), anhydrous dichloromethane (30mL) Sequentially added to a 250mL eggplant-shaped bottle, slowly added di-tert-butyl dicarbonate (8.51g, 39.0mmol) diluted with anhydrous dichloromethane (50mL) dropwise under stirring, and the reaction was continued at room temperature for 4 hours after the drop was completed. Stop the reaction, add water to separate the layers, extract the aqueous phase with dichloromethane (3×50mL), combine the organic phases, wash with tap water (3×50mL) and saturated brine (30mL) successively, evaporate the organic phase to dryness under reduced pressure, and carry out silica gel Separation and purification by column chromatograph...
Embodiment 2
[0217] Example 2: 1-(6-methoxy-4,4-dimethyl-3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)butane Synthesis of yl-1-ones
[0218]
[0219] Step 1) 1-(6-Methoxy-4,4-dimethyl-3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)butyl Synthesis of -1-one
[0220] 6-Methoxy-4,4-dimethyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (127mg, 0.55mmol), anhydrous N, N-dimethylformamide (15 mL), N,N-diisopropylethylamine (0.39 mL, 2.2 mmol) and 2-(7-azobenzotriazole)-N,N,N' ,N'-Tetramethyluronium hexafluorophosphate (323mg, 0.82mmol) was successively added into a 100mL eggplant-shaped flask, and at -10°C, anhydrous N,N-dimethylformamide (5mL ) dissolved n-butyric acid (0.06mL, 0.66mmol), after the dropwise reaction was continued for 20 minutes, it was transferred to room temperature and reacted for 10 hours. Stop the reaction, add water to separate the layers, extract the aqueous phase with dichloromethane (3×30mL), combine the organic phases, wash with tap water (3×50mL) and saturated brine (30...
Embodiment 3
[0224] Embodiment 3: the synthesis of N-((1-(5-methoxy-1H-indol-3-yl) cyclopropyl) methyl) propanamide
[0225]
[0226] Step 1) Synthesis of tert-butyl-3-(1-cyanocyclopropyl)-5-methoxy-1H-indole-1-carboxylate
[0227] The title compound of this step was prepared by referring to the method described in step 2 of Example 1, that is, tert-butyl-3-(cyanomethyl)-5-methoxy-1H-indole-1-carboxylate (5g, 17.46mmol), 1,2-dibromoethane (2.28mL, 26.9mmol), 60% sodium hydride (1.75g, 43.8mmol) were prepared by reacting in anhydrous dimethyl sulfoxide (45mL), and the crude product was purified by silica gel Separation and purification by column chromatography (petroleum ether / ethyl acetate (v / v)=100 / 1) gave the title compound (light yellow liquid, 1.66 g, 30.4%).
[0228] MS(ESI,pos.ion)m / z:313.3[M+H] + ;
[0229] 1 H NMR (600MHz, CDCl 3 )δ (ppm): 7.98 (s, 1H), 7.43 (s, 1H), 7.20 (d, J = 2.4Hz, 1H), 6.96 (dd, J = 9.0, 2.4Hz, 1H), 3.89 (s, 3H), 1.67~1.65(m, 2H), 1.64(s, 9H), 1.33...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com