Composition for improving contact dermatitis containing pellitorine as an active ingredient
A technology of contact dermatitis and composition, applied in the field of contact dermatitis improvement composition, capable of solving imperfections and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1. Isolation of pellitorine compound from Caulis japonica extract
[0068] After pulverizing the dried Caulis chinensis (10.2kg), extracting with 80% acetone at room temperature for 3 times and filtering, the extract was concentrated under reduced pressure to obtain 340g of extract. After the extract was concentrated under reduced pressure, 70% methanol-hexane (MeOH-Hexane), 70% methanol-chloroform (MeOH-Chloroform) was used to carry out solvent fractionation, and the fractionation was divided into 3 layers (hexane layer, chloroform layer, 70 % methanol layer). Among them, silica gel (Silica gel) column chromatography was used for the hexane layer, and the concentration of the solvent was increased according to hexane-ethyl acetate = 1:0 to 0:1. After performing TLC, it was fractionated into 17 layers. Among them, for Fr.9 The layer is subjected to DaiSogel ODS-B and medium pressure liquid chromatography (MPLC) (60% MeOH→100% MeOH, gradient system) column chr...
Embodiment 2
[0069] Example 2. Identification of the structure of pellitorine compound
[0070] Determination of the pellitorine compound obtained in Example 1 1 H and 13 C-nuclear magnetic resonance spectrum (NMR spectrum), its result is as follows:
[0071] ① 1 H-NMR (600MHz, CDCl 3 )
[0072] :δ H 7.18(1H,dd,J=10.8,15.0Hz,H-3'),6.12(1H,dd,J=10.8,15.0Hz,H-4'),6.05(1H,dt,J=6.6,15.0Hz ,H-5'),5.90(1H,br s,N-H),5.81(1H,d,J=15.0Hz,H-2'),3.15(2H,t,J=6.6Hz,H-1), 2.13(2H,m,H-6'),1.80(1H,m,H-2'),1.41(2H,m,H-7'),1.29(4H,m,H-8',9') ,0.92(6H,d,J=6.6Hz,CH 3 -3,4),0.88(3H,t,J=7.2Hz,CH 3 -10')
[0073] ② 13 C-NMR (150MHz, CDCl 3 )
[0074] :δ c 166.5(C-1'), 143.0(C-5'), 141.1(C-3'), 128.2(C-4'), 121.8(C-2'), 46.9(C-1), 32.8(C -6'),31.3(C-8'),28.5(C-7'),28.5(C-2),22.4(C-9'),20.1(C-3,4),13.9(C-10 ')
[0075] The pellitorine compound obtained in Example 1 was determined to be (E,E)-N-(2-methylpropyl)2,4-decadienamide represented by the following Chemical Formula 1 from the above resul...
Embodiment 3
[0078] Example 3. Evaluation of the effect of pellitorine compound on regulating immune cell activity
[0079] 3-1. Isolation of splenocytes
[0080] In order to perform the experiments described below, splenocytes need to be isolated from experimental animals. That is, 6-7 week-old Balb / C mice were used as experimental animals provided by Orient Bio (Seongnam, Korea) Co., Ltd. The experimental animals were kept at 22-24° C. and the humidity was 50%, and sufficient feed and water were supplied. Spleens excised from Balb / C mice were sufficiently crushed with a mesh, and connective tissue and the like were removed with a cell strainer (SPL, Korea), followed by centrifugation to obtain splenocytes. 10 ml of ACK solvent was added to the obtained splenocytes, reacted at room temperature for 5 minutes, and after red blood cells were removed, the splenocytes were separated by centrifugation twice.
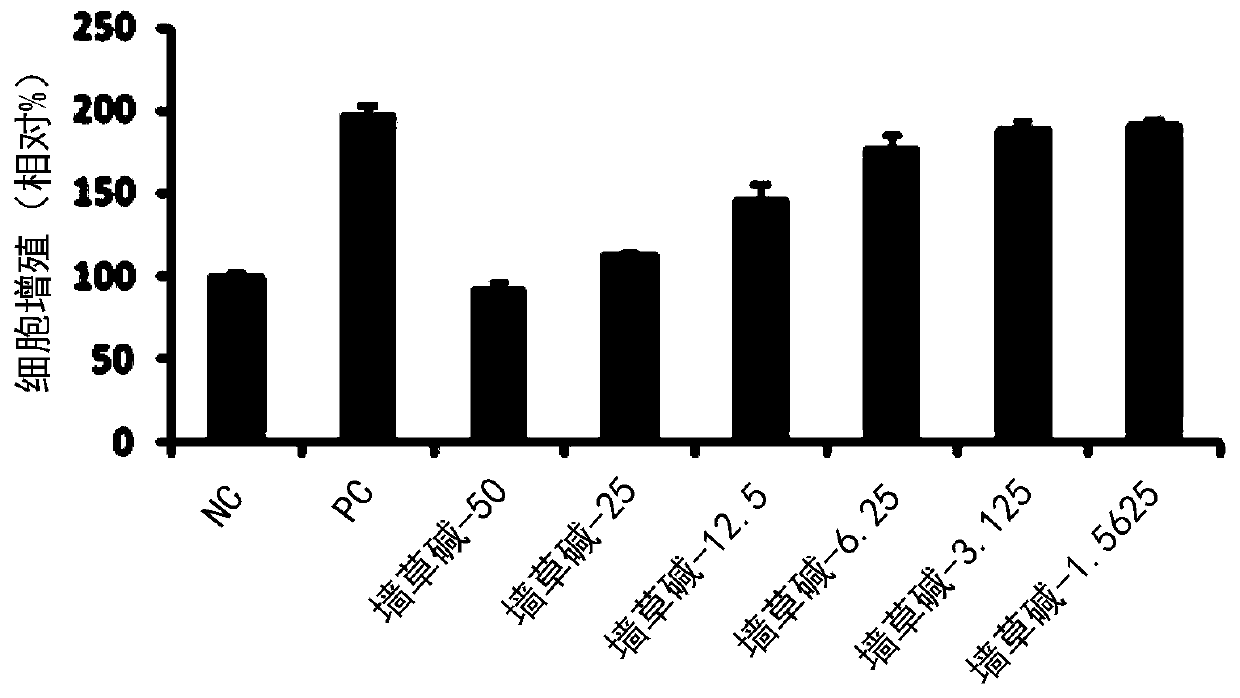
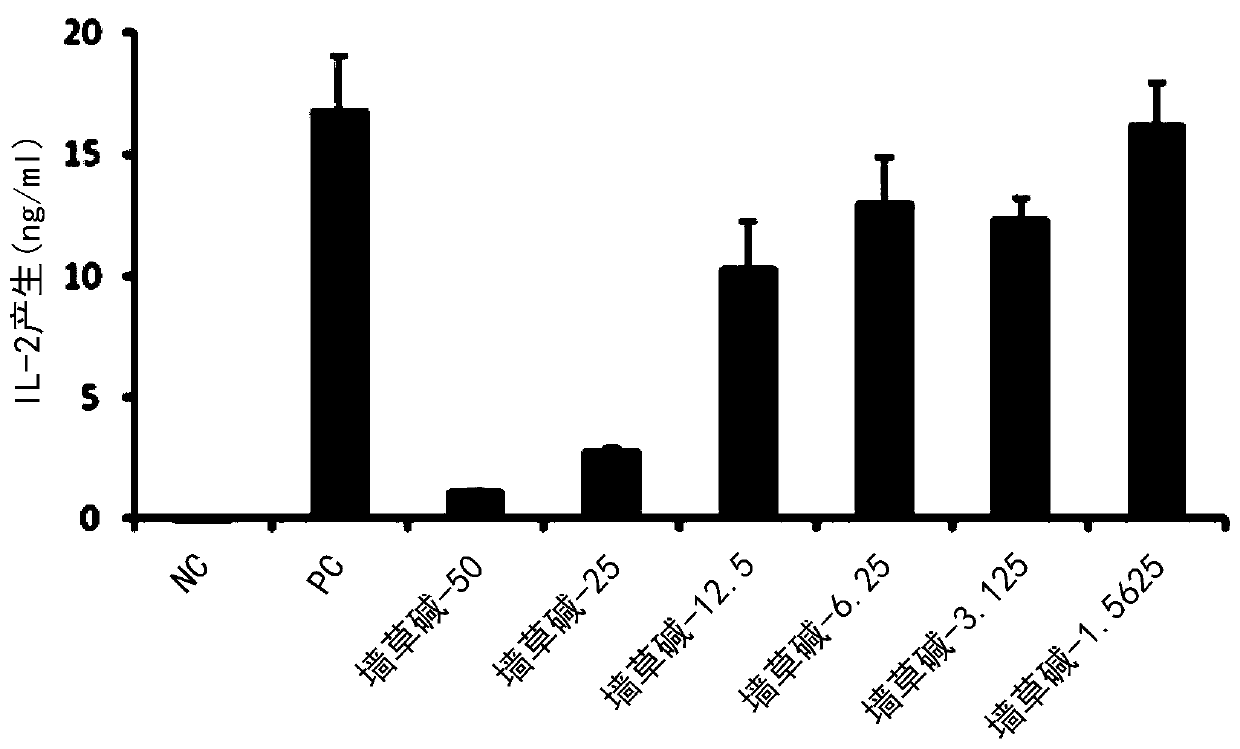
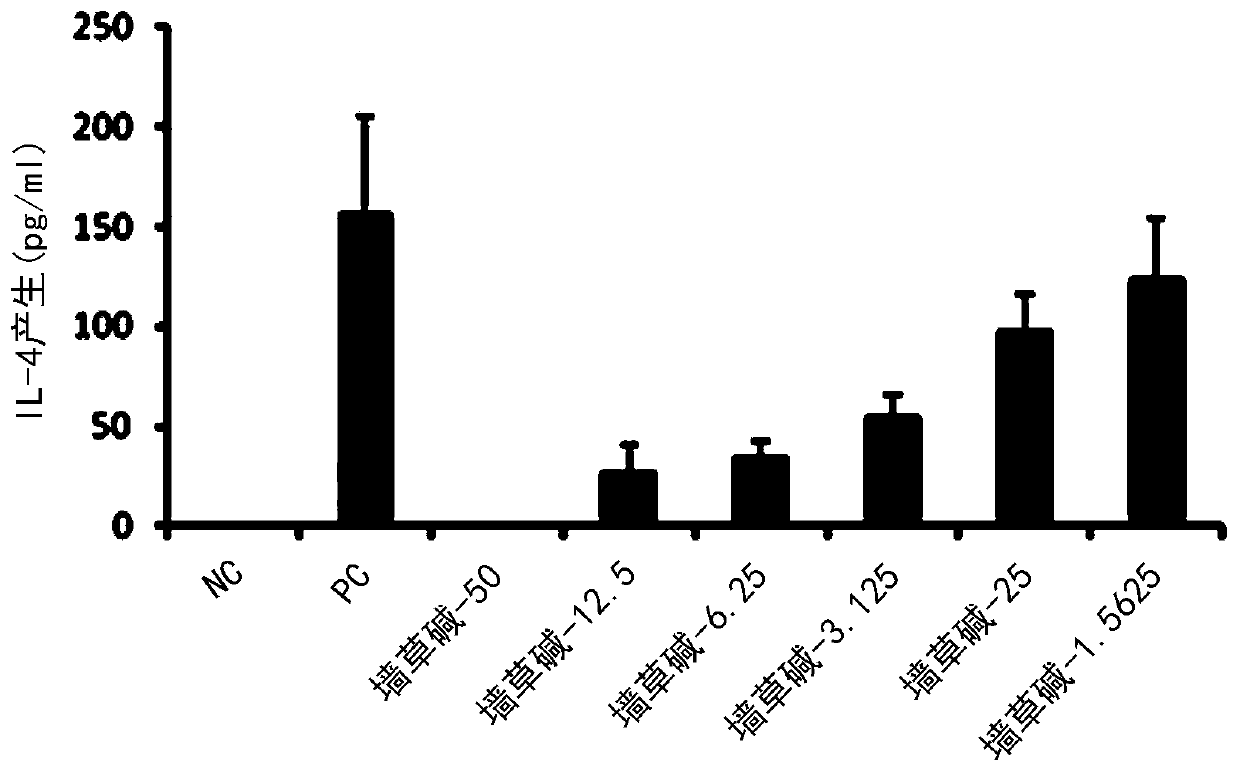
[0081] 3-2. Determination of the ability of pellitorine compounds to inhibit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com