Preparation method of anhydrous lanthanum bromide
An aqueous lanthanum bromide and ammonium bromide technology, applied in the field of preparation of anhydrous lanthanum bromide, can solve the problems of restricting the development of lanthanum bromide scintillation crystals, demanding content control, difficult process control and the like, and achieving an easy preparation process. Control, low production cost, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 6.52g of lanthanum oxide and 23.5g of ammonium bromide were mixed uniformly and then added to the corundum crucible. The mixture was first held at 400°C for 2.0 hours and then at 550°C for 1.0 hour. In this way, anhydrous lanthanum bromide is obtained. In this embodiment, the mass ratio of ammonium bromide and lanthanum oxide is 3.60:1, the mixture of lanthanum oxide and ammonium bromide is the covering, and the covering thickness is 7 mm.
[0063] According to the above measurement method, the obtained anhydrous lanthanum bromide was characterized, and the results are as follows:
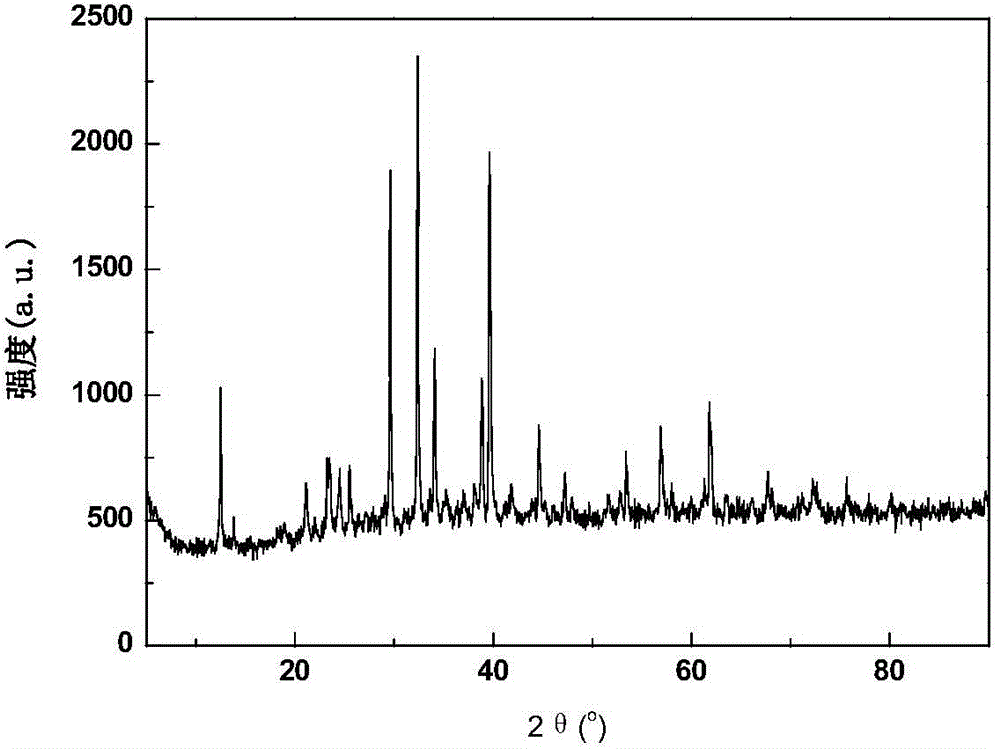
[0064] Perform XRD phase analysis on the sample composition, the composition is anhydrous LaBr 3 , See attached figure 1 .
[0065] The precipitate after the sample was dissolved in water was titrated, and the purity of the anhydrous lanthanum bromide was determined to be 99.58%.
[0066] The moisture content in the sample is determined, and the moisture content in the sample is 0.08%.
Embodiment 2
[0068] 3.26g lanthanum oxide and 11.75g ammonium bromide were mixed uniformly and then added to the corundum crucible. Then, 10 mm thick aluminum oxide was coated on the mixture of lanthanum oxide and ammonium bromide. The mixture was first held at 350°C for 2.0 hours and then at 550°C for 1.0 hour. In this way, the upper layer of alumina with obvious stratification and the lower layer of anhydrous lanthanum bromide are obtained. In this embodiment, the mass ratio of ammonium bromide and lanthanum oxide is 3.60:1, aluminum oxide is the covering, and the covering thickness is 10 mm.
[0069] According to the above measurement method, the obtained anhydrous lanthanum bromide was characterized, and the results are as follows:
[0070] Perform XRD phase analysis on the sample composition, the composition is anhydrous LaBr 3 .
[0071] The precipitate after the sample was dissolved in water was titrated, and the purity of anhydrous lanthanum bromide was determined to be 99.60%.
[007...
Embodiment 3
[0074] 13.04g lanthanum oxide and 47.00g ammonium bromide were mixed uniformly and then added to the corundum crucible. Then, 13mm thick aluminum oxide was coated on the mixture of lanthanum oxide and ammonium bromide. The mixture was first held at 400°C for 2.5 hours and then at 600°C for 1.0 hour. In this way, the upper layer of alumina with obvious stratification and the lower layer of anhydrous lanthanum bromide are obtained. In this embodiment, the mass ratio of ammonium bromide and lanthanum oxide is 3.60:1, alumina is the covering, and the covering thickness is 13 mm.
[0075] According to the above measurement method, the obtained anhydrous lanthanum bromide was characterized, and the results are as follows:
[0076] Perform XRD phase analysis on the sample composition, the composition is anhydrous LaBr 3 .
[0077] The precipitate after the sample was dissolved in water was titrated, and the purity of anhydrous lanthanum bromide was determined to be 99.69%.
[0078] The mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com