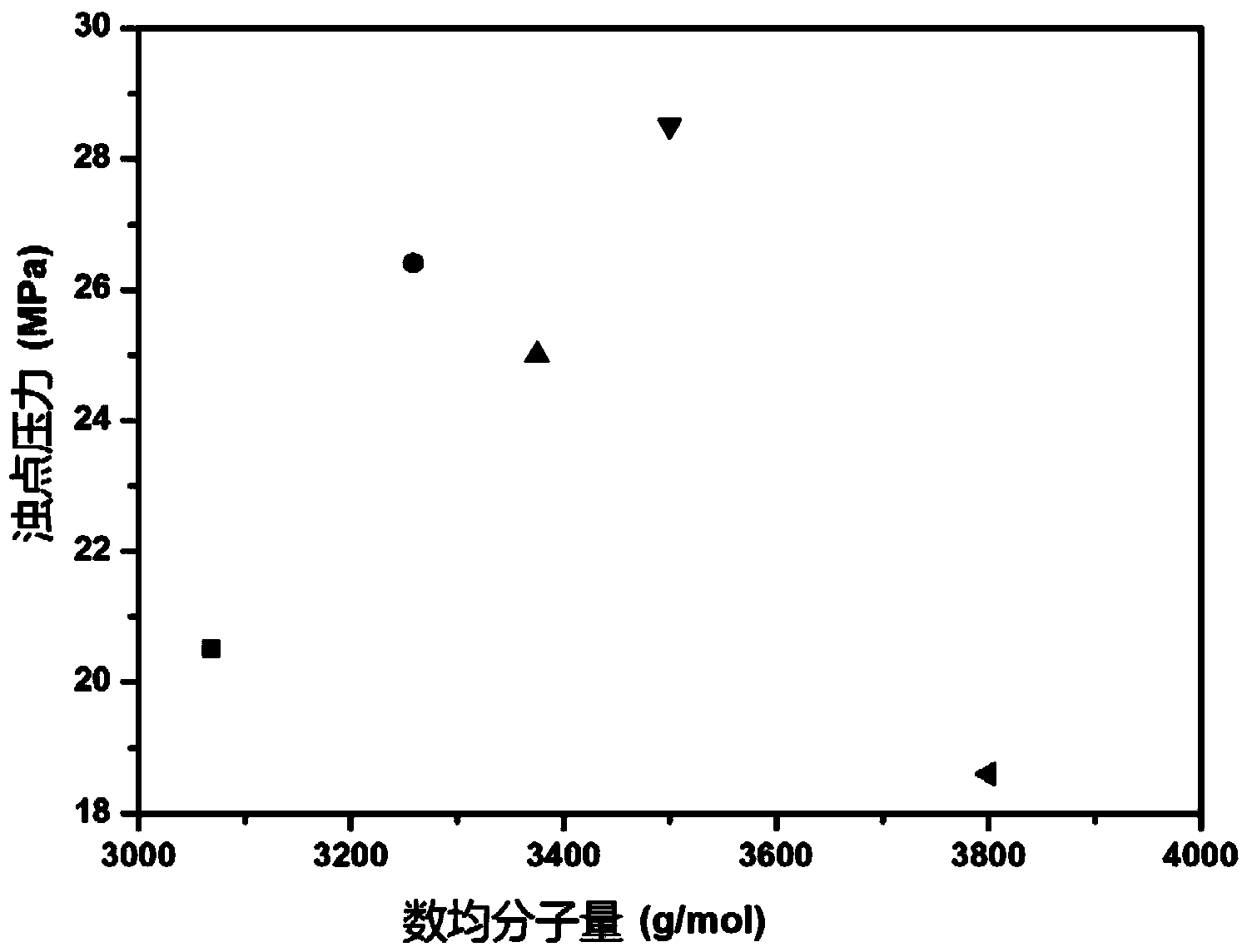
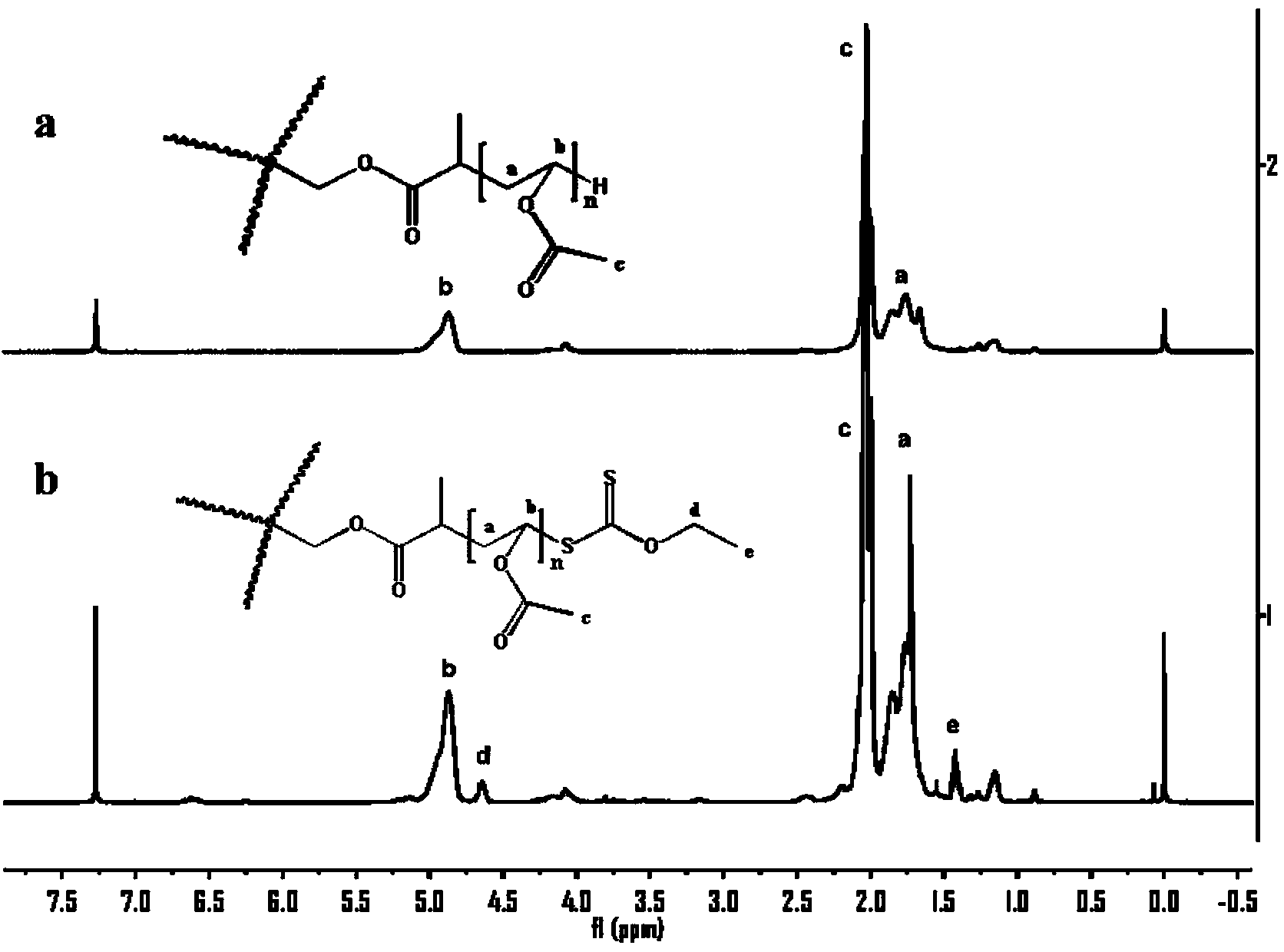
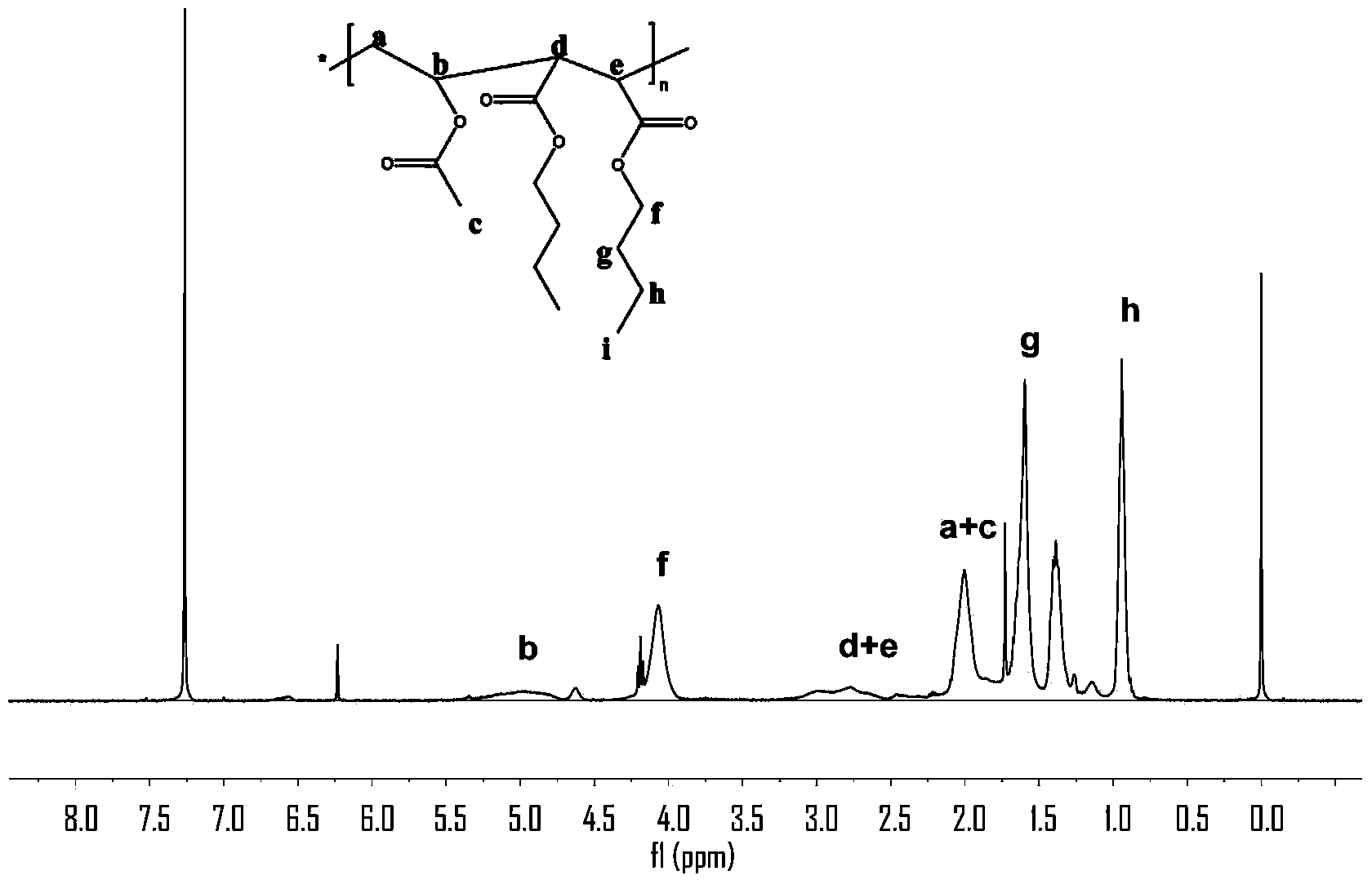
Topological structure polymer, and preparation method and application thereof
A topology and polymer technology, applied in the preparation of organic compounds, carboxylic acid ester preparation, drilling composition, etc., can solve the adverse effects of polymer physical and chemical properties, dithioester group or xanthate The group is not easy to remove, the polymer is not easy to low molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Step a), take tris(hydroxymethyl)ethane (5.4g), chloroform (50ml), pyridine (8ml) in the reactor, place the reactor in an ice-water bath, slowly add 2-bromopropionyl bromide ( 29.1 g), remove the ice-water bath after the dropwise addition, seal the reactor, and stir and react at room temperature for 48-72 hours. The organic phase was washed with 50ml of 25vt% dilute hydrochloric acid and 100ml of 10wt% sodium bicarbonate solution and dried over anhydrous magnesium sulfate for several hours. The solvent was distilled off under reduced pressure to obtain a white solid powder.
[0112] Step b). Take the three-arm brominated precursor (20g), chloroform (100ml) and excess potassium ethyl xanthate (40g) in the reactor at room temperature and stir for 72h to 96h, then filter The excess suspended potassium ethyl xanthate was washed 5 times with 100 ml of chloroform, and the solvent was distilled off under reduced pressure. Obtain 8.7g three-arm topology xanthates (its structu...
Embodiment 2
[0114] Step a), take pentaerythritol (5.72g), chloroform (50ml), and pyridine (8ml) in the reactor, place the reactor in an ice-water bath, slowly add 2-bromopropionyl bromide (30.0g), dropwise Finally, the ice-water bath was removed, the reactor was sealed, and the reaction was stirred at room temperature for 48-72h. The organic phase was washed with 60ml of 25vt% dilute hydrochloric acid and 120ml of 10wt% sodium bicarbonate solution and dried over anhydrous magnesium sulfate for several hours. The solvent was distilled off under reduced pressure to obtain a white solid powder.
[0115] Step b), take the product four-arm brominated precursor (20g) of the above step a), chloroform (10ml), excess potassium ethyl xanthate (4g) in the reactor at room temperature and stir for 72h-96h, filter the excess The suspended potassium ethyl xanthate was washed 6 times with 120 ml of chloroform, and the solvent was distilled off under reduced pressure. Obtain 9.2g four-arm topology xanth...
Embodiment 3
[0117] Take vinyl acetate (18ml), tetrahydrofuran (4ml), helium diisobutyronitrile (0.6065g) and 1.5g of the three-arm topoxanthate prepared in Example 1, and mix them uniformly. Before the reaction, the reactor was evacuated and dry nitrogen was introduced three times, and the reactor was sealed. The reactor was stirred and reacted at a constant temperature of 60°C for 30 minutes, and the product obtained from the reaction was dissolved in 5ml of tetrahydrofuran, precipitated in 200ml of ice-n-hexane, filtered with suction, and the precipitate was vacuum-processed in a 40°C oven for 48 hours to obtain 2.8g with a molecular weight of 956 and a molecular weight distribution of 1.04 polyvinyl acetate.
[0118] Take polyvinyl acetate (2.46mmol), tetrahydrofuran (3ml), isopropanol (6ml) and dilauroyl peroxide (8mmol) synthesized in the previous step reaction in the reactor, vacuumize and feed dry nitrogen repeatedly three times, seal reactor. React at 70°C for 6 hours, dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com