LiFePO4 positive electrode material modified jointly by doping and coating and preparation method thereof
A positive electrode material, lithium iron phosphate technology, applied in the field of electrochemical power supply, can solve the problems of poor electrical conductivity, low compaction density, and high price, and achieve excellent electrochemical performance, less harmful gas emissions, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
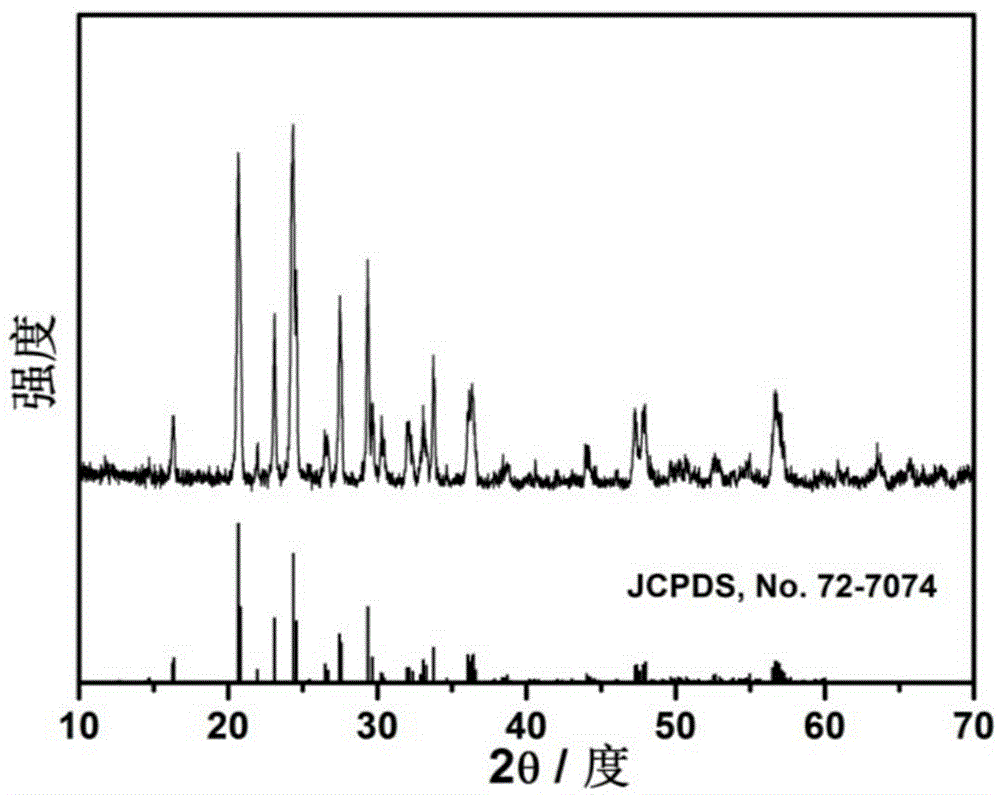
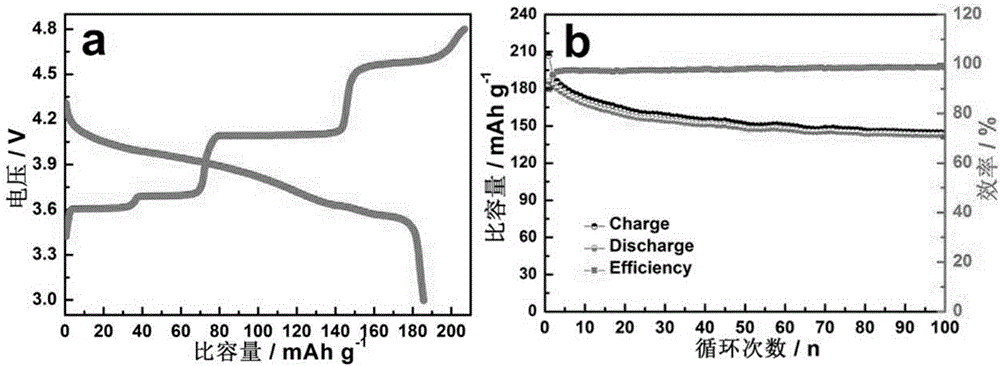
[0022] Si(OC 2 h 5 ) 4 Add in alcohol and stir for 1h, then add the powder obtained in Comparative Example 1, wherein, lithium source, vanadium source, iron source, phosphorus source, Si(OC 2 h 5 ) 4 The ratio of the amount of substances is 3.05:1.95:0.05:3:0.18. After ultrasonication for 2 hours, the solvent was evaporated under an infrared lamp, then sintered at 500-600°C for 5 hours in a nitrogen atmosphere, and sieved to obtain the sample LVFP / C-Si. The active material LVFP / C-Si, acetylene black, and polyvinylidene fluoride (PVdF) were adjusted into a slurry in N-methylpyrrolidone (NMP) medium at a mass ratio of 75:15:10, and coated on aluminum foil. After drying, film punching and film pressing, the working electrode is made. With metal lithium foil as the counter electrode, polypropylene film as the separator, 1M LiPF 6 / (EC+DMC)(1:1) is assembled into a battery with electrolyte solution for constant current charge and discharge test, and the voltage range is betw...
Embodiment 2
[0024] Will (CH 3 COO) 2 Co 4H 2 O is added in alcohol and stirred for 1h to dissolve, then add the powder obtained in Comparative Example 1, wherein lithium source, vanadium source, iron source, phosphorus source, (CH 3 COO) 2 Co 4H 2 The ratio of the amount of O to the substance is 3.05:1.95:0.05:3:0.06. After ultrasonication for 2h, the solvent is evaporated under the infrared lamp, and then sintered at 500-600°C for 5h in a nitrogen atmosphere, and sieved to obtain the sample LVFP / C-Co . The active material LVFP / C-Co, acetylene black, and polyvinylidene fluoride (PVdF) were adjusted into a slurry in N-methylpyrrolidone (NMP) medium at a mass ratio of 75:15:10, and coated on aluminum foil. After drying, film punching and film pressing, the working electrode is made. With metal lithium foil as the counter electrode, Celgard2400 as the diaphragm, 1M LiPF 6 / (EC+DMC)(1:1) is assembled into a battery with electrolyte solution for constant current charge and discharge tes...
Embodiment 3
[0026] A certain amount of (CH 3 COO) 2 Ni·4H 2 O is added in alcohol and stirred for 1h to dissolve, then add the powder obtained in Comparative Example 1, wherein lithium source, vanadium source, iron source, phosphorus source, (CH 3 COO) 2 Ni·4H 2 The amount ratio of O is 3.05:1.95:0.05:3:0.14. After ultrasonication for 2h, the solvent is evaporated under the infrared lamp, and then sintered at 500-600°C for 5h in a nitrogen atmosphere, and sieved to obtain the sample LVFP / C-Ni . The active material LVFP / C-Ni, acetylene black, and polyvinylidene fluoride (PVdF) were adjusted into a slurry in N-methylpyrrolidone (NMP) medium at a mass ratio of 75:15:10, and coated on aluminum foil. After drying, film punching and film pressing, the working electrode is made. With metal lithium foil as the counter electrode, Celgard2400 as the diaphragm, 1M LiPF 6 / (EC+DMC)(1:1) is assembled into a battery with electrolyte solution for constant current charge and discharge test, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com