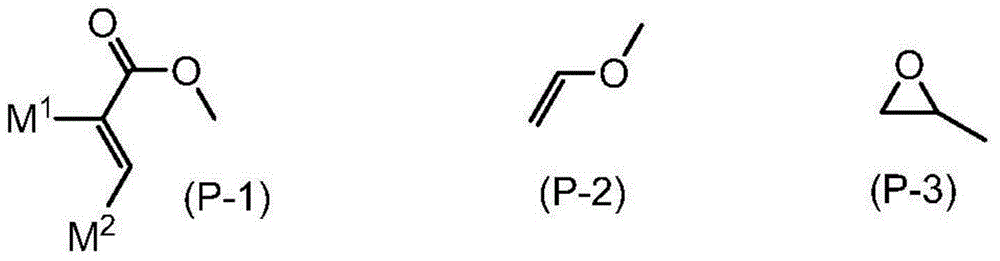Polymerizable compound, liquid crystal composition, and liquid crystal display element
A liquid crystal composition and compound technology, applied in liquid crystal materials, organic chemistry, nonlinear optics, etc., can solve the problems of decreased polymerization reactivity and achieve the effects of large contrast, wide temperature range, and high voltage retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0206] The preparation of the liquid crystal composition is carried out by a method such as dissolving essential components together at a temperature higher than room temperature. Depending on the application, additives may be added to the composition. Examples of additives are optically active compounds, antioxidants, ultraviolet absorbers, light stabilizers, heat stabilizers, defoamers, and the like. Such additives are well known to those skilled in the art and are described in the literature.
[0207] The optically active compound has the effect of preventing reverse twist by creating a helical structure in liquid crystal molecules and imparting a necessary twist angle. By adding optically active compounds, the helical pitch can be tuned. For the purpose of adjusting the temperature dependence of the helical pitch, two or more optically active compounds may be added. Preferable examples of the optically active compound include the following compound (Op-1) to compound (O...
Embodiment 1
[0265] [Example 1] Synthesis of Compound No.26
[0266]
[0267] Step 1
[0268] (1,1'-biphenyl)-4,4'-diol (T-1) (200g, 1.07mol) was dissolved in DMF (2000ml), and tetrabutylammonium bromide (34.49g, 0.107mol), potassium carbonate (355g, 2.57mol) and 2-bromo-1,1-diethoxyethane (506g, 2.57mol), heated to reflux for 5 hours. After cooling the reaction solution to room temperature, it was poured into water (1000ml) and extracted with toluene (500ml×3). The extract was washed with saturated brine (500 ml×3), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (toluene:ethyl acetate=19:1 by volume ratio), and then recrystallized from heptane to obtain compound (T-2) (434g, 1.07mol , 100%).
[0269] Step 2
[0270] After compound (T-2) (434 g, 1.07 mol) was dissolved in chloroform (4000 ml), trifluoroacetic acid ((918 ml, 12.36 mol)) was added dropwise at room temperature. After 16 hou...
Embodiment 2
[0275] [Example 2] Synthesis of Compound No.1
[0276]
[0277] Compound No. 1 was obtained by using acrylic anhydride and sodium acrylate in the third step in the same manner as the method described in Example 1.
[0278] 1 H-NMR (CDCl 3 ; δppm): 7.52 (d, 4H), 7.12 (d, 4H), 6.98 (d, 2H), 6.58 (dd, 2H), 6.26 (dd, 2H), 6.19 (d, 2H), 5.97 (dd, 2H).
[0279] Melting point: 121.1°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com