Method for enriching glycopeptide by phenylboronic acid material
A phenylboronic acid and enrichment technology, applied in the field of separation and purification, can solve problems such as reducing selectivity, and achieve the effects of improving detection limit, wide versatility and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The preparation process of monolayer phenylboronic acid modified silica spheres was L-aspartic acid (4g, 30mmol), K2CO3 (18g, 130mmol), CuSO4·5H2O (0.075g, 0.3mmol), 2-oxazole-1-sulfonyl alkene Nitride (8.5g, 40.6mmol), CH3OH (100mL) and spherical silica gel (20g) with a particle size of 5μm were added to a 250mL round-bottomed flask, and the mixture was stirred at room temperature for 9h, then concentrated and dried by centrifugation to obtain α- alkene Nitrogen L-Aspartate. Then, copper acetate (0.4 g, 2 mmol) and sodium ascorbate (0.8 g, 4 mmol) aqueous solution (100 mL) were added to α-azide L-aspartic acid, and stirred at room temperature for 114 h. The obtained product was successively washed with methanol, water, 10% EDTA aqueous solution, water, and methanol twice, 100ml each time, and vacuum-dried at room temperature for 12 hours to obtain a single-layer phenylboronic acid-modified silicon sphere.
[0063] The preparation process of multilayer phenylboronic ac...
Embodiment 1
[0067] Put 1 mg of phenylboronic acid-modified silicon spheres into the extraction column, equilibrate the column with 30 L of 50 mM ammonium bicarbonate solution containing 80% acetonitrile by volume concentration; digest 10 L of 100 g / mL standard glycoprotein horseradish peroxidase with trypsin The solution was spin-dried, dissolved in 30L of 50mM ammonium bicarbonate solution containing 80% acetonitrile by volume, and loaded onto the extraction column;
[0068] Wash with 30L of 50mM ammonium bicarbonate solution containing 80% acetonitrile to remove non-glycopeptides; repeat the washing process twice;
[0069] Then, 20 L of 50% acetonitrile (pH3) aqueous solution was used to elute the glycopeptide, and the elution process was repeated twice; the enriched glycopeptide fractions were combined and analyzed by mass spectrometry.
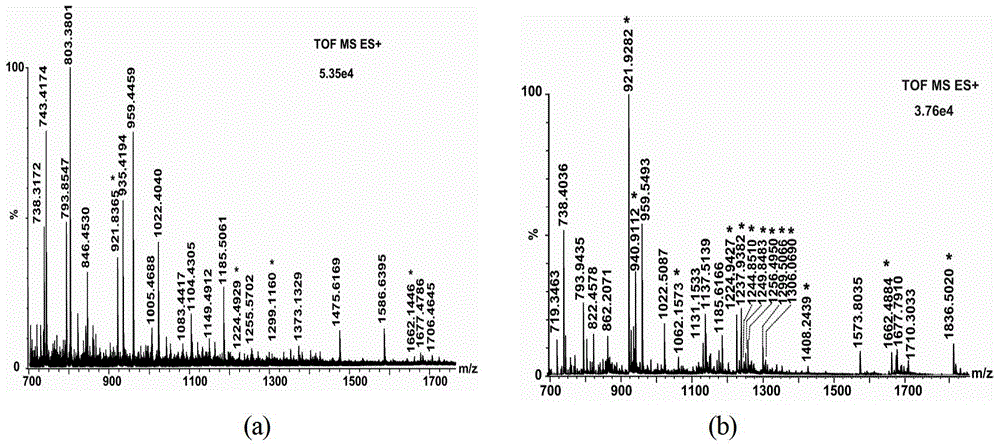
[0070] Depend on figure 1 It can be seen that the multilayer phenylboronic acid-modified silicon spheres are specific for the enrichment of glycopep...
Embodiment 2
[0072] Disperse 1 mg of phenylboronic acid-modified silicon spheres with 100 L of 50 mM ammonium bicarbonate solution containing 80% acetonitrile by volume, centrifuge, discard the upper layer solution, and collect the precipitate; 10 L of 100 g / mL standard glycoprotein horseradish peroxidase trypsin After the enzymolysis solution was spin-dried, dissolve it in 100L of 50mM ammonium bicarbonate solution containing 80% acetonitrile by volume, mix it with phenylboronic acid-modified silica gel material, incubate for 0.5-120 minutes (specifically, 30 minutes), centrifuge, and discard the upper layer solution. Collect the precipitate; the centrifuged phenylboronic acid-modified silica gel material is mixed with 100L of 50mM ammonium bicarbonate solution containing 80% acetonitrile and incubated for 0.5-120 minutes, centrifuged, the upper layer solution is discarded, and the precipitate is collected; repeat this step twice;
[0073] After centrifugation, the phenylboronic acid-modif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com