Continuous preparation method of statin pharmaceutical intermediate namely (R)-3-hydroxyethyl glutarate
A technology of ethyl hydroxyglutarate and ethyl hydroxybutyrate, applied in the field of organic drug synthesis, can solve the problems of no nitrilase, difficult screening of biological catalysts, etc., achieve broad industrial production prospects, reduce reaction purification and separation steps, the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
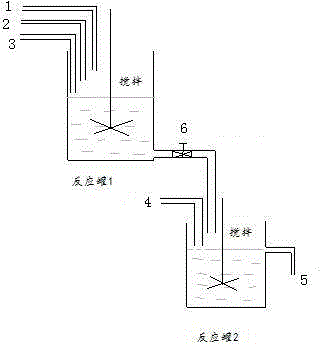
[0023] Example 1: Such as figure 1 As shown, the preparation device of the present invention comprises a reaction tank 1 and a reaction tank 2, and a stirring device is provided in the reaction tank 1 and 2, and the reaction tank 1 and the reaction tank 2 are connected by a pipeline with a flow regulating valve 6, and the reaction Tank 1 is provided with substrate addition pipeline 1, pH control pipeline 2 and recombinant halohydrin dehalogenase addition pipeline 3, and reaction tank 2 is provided with recombinant nitrilase addition pipeline 4 and product outlet pipe.
Embodiment 2
[0024] Example 2: The actual implementation process of the preparation method of recombinant halohydrin dehalogenase HHDH and nitrilase NIT of the present invention is as follows:
[0025] Inoculate single colonies of recombinant Escherichia coli purchased on the market containing recombinant halohydrin dehalogenase and nitrilase genes into liquid LB medium containing kanamycin resistance, shake at 37°C and shake at 200rpm Cultivate for 7 hours, inoculate the cultured bacterial liquid into the liquid LB medium containing kanamycin resistance, and culture at 37°C with shaking at 200 rpm until OD 600 When the value reaches 1.0, add the inducer isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 1mM, continue to cultivate at 28°C for 20 hours, collect the bacteria by centrifugation at 4°C, and add physiological saline to wash The cells were collected twice by centrifugation again, and the cells were suspended with 3 times the volume of triethanolamine buffer ...
Embodiment 3
[0026] Example 3: The actual implementation process of the method preparation method for the continuous preparation of statin drug intermediate (R)-3-hydroxyglutarate ethyl ester of the present invention is as follows:
[0027]Add 45g of substrate (S)-CHBE and 5g of 30% sodium cyanide phosphoric acid solution at pH 7.0 in reaction tank 1 with a volume of 500ml, add 230ml of deionized water, start stirring, and adjust the temperature to 50°C; in 500ml reaction tank 2 , the volume below the overflow port is 400ml, initially add 50ml of deionized water and 5g of crude nitrilase enzyme solution to the lowest stirring blade, start stirring, and adjust the reaction temperature to 25°C. Add 5g of halohydrin dehalogenase crude enzyme liquid into reaction tank 1 to start the reaction. During the reaction, use 30% NaCN solution to control the pH of the system to 7.0. After 6h, use gas chromatography (GC) to detect that the conversion rate of the substrate is 97.3 %, start to add the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com