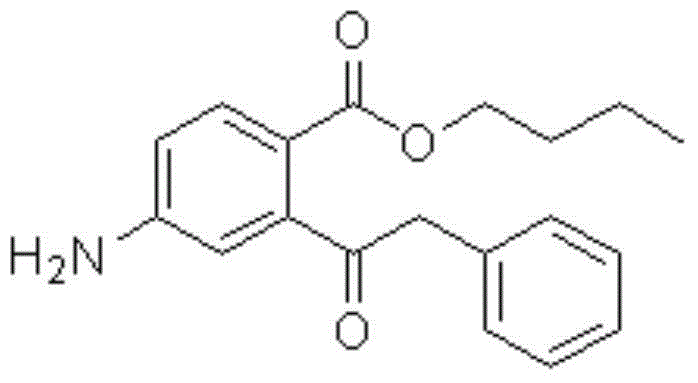
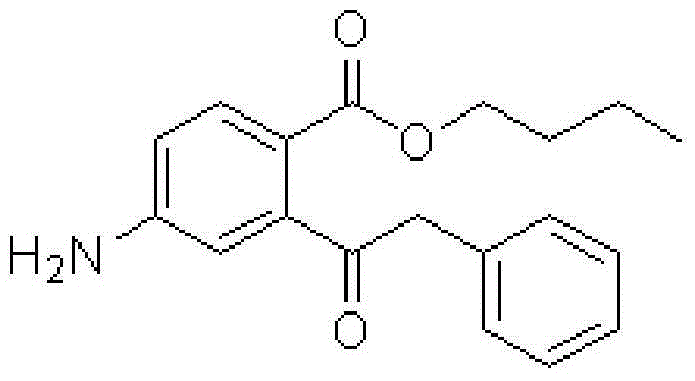
Benzyl butyl phthalate hapten derivative, preparation method of benzyl butyl phthalate hapten derivative and detection method of benzyl butyl phthalate
A technology of butyl benzyl phthalate and nitrobutyl benzyl phthalate, applied in the field of organic synthesis, can solve the problem of not having immunogenicity and not involving butyl benzyl phthalate hapten Derivatives, whole antigens and reports of immunoassays, etc., to achieve the effect of simple synthesis method and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of Butylbenzyl 4-Aminophthalate
[0057] Mix 9.655g of 4-nitrophthalic anhydride and 5.125mL of anhydrous n-butanol into a three-neck flask with a reflux condenser, a thermometer and a rotor, and raise the temperature to 120°C under stirring to completely dissolve it into Colorless transparent liquid, cooled, stirred and refluxed at 110°C for 1.0 hour, then cooled the reaction solution to 80°C, neutralized with 40% sodium hydroxide solution, and adjusted the pH of the solution to 8-8.5 with 20% sodium carbonate solution . Add 6g of polyethylene glycol 600 under stirring, raise the temperature to 100°C and slowly add 26mL of benzyl chloride dropwise. After reacting at 100°C for 4 hours, the crude product is separated and purified by column chromatography after cooling to obtain a yellow oily liquid which is 4 -Butylbenzyl nitrophthalate.
[0058] Dissolve 0.608g of 4-nitrobutylbenzyl phthalate into 20mL of benzene, add some zinc powder; The molar ratio of 4-...
Embodiment 2
[0082] Synthesis of butylbenzyl 4-aminophthalate:
[0083] Mix 19.31g of 4-nitrophthalic anhydride and 23.30mL of anhydrous n-butanol into a three-necked flask with a reflux condenser, a thermometer and a rotor, and raise the temperature to 110°C under stirring to completely dissolve Color transparent liquid, cooled, stirred and refluxed at 105°C for 2.0 hours, then cooled the reacted solution to 70°C, neutralized with 30% sodium hydroxide solution, and adjusted the pH of the solution to 8-8.5 with 20% sodium carbonate solution. Add 10g of polyethylene glycol 800 under stirring, raise the temperature to 115°C and slowly add 25.3mL of benzyl chloride dropwise, react at 100°C for 2.5 hours, the crude product is separated and purified by column chromatography after cooling to obtain a yellow oily liquid which is 4-Nitrobutylbenzyl phthalate, yield 71%.
[0084] Dissolve 0.525g of 4-nitrobutylbenzyl phthalate into 15mL of benzene, add some magnesium powder; The molar ratio of 4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com