Glaucocalyxin A thiazole derivative, preparation method and application thereof
A technology of calyxine and derivatives, applied in the field of medical invention, can solve the problems of low polarity, poor water solubility, drug administration and the like, and achieve the effects of improving water solubility, excellent activity and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of 2-bromocalyxin
[0071] Weigh 166mg (0.5mmol) cylindrin and dissolve it in 5mL THF, put it in an ice bath, then dissolve 160mg (0.5mmol) tribromopyridinium in 1mL THF, slowly add it to the reaction system dropwise after ice bathing, add 30mL distilled water after 30min Quench the reaction, extract with dichloromethane, 30 mL each time, extract 3 times, combine the extracts, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, separate by silica gel column chromatography, petroleum ether-ethyl acetate (2:1) was eluted to obtain 145.5 mg of white solid (C-2 epimer) with a yield of 70.8%. mp.203-205℃
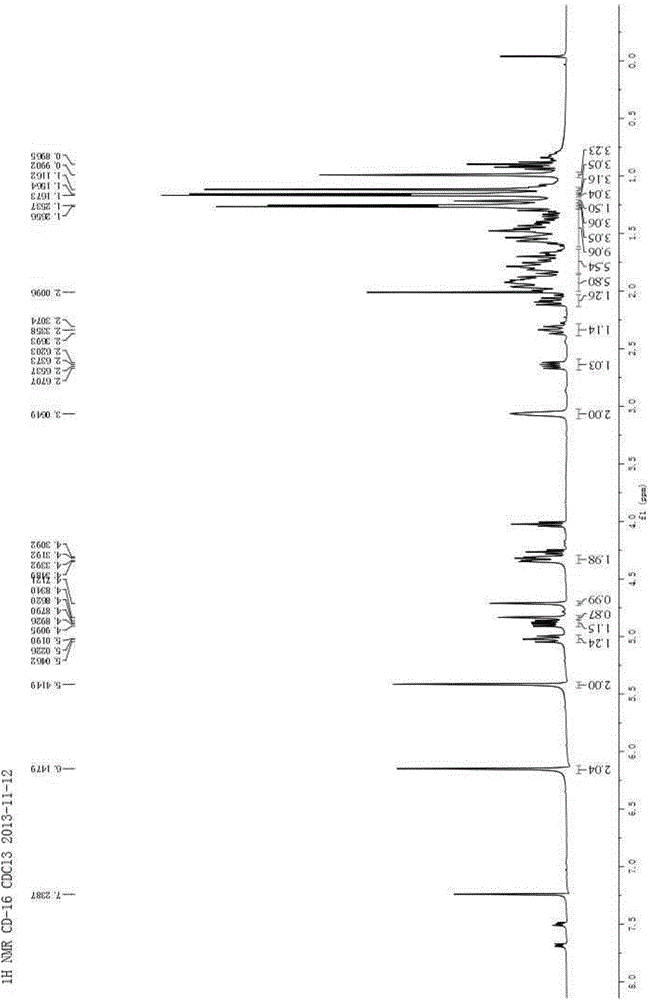
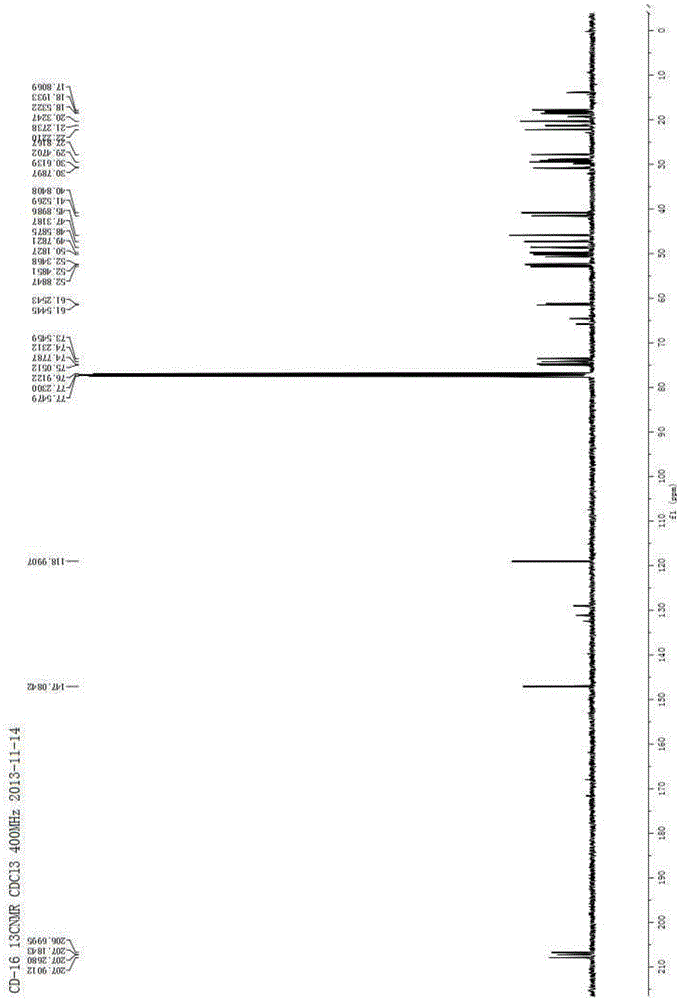
[0072] Isomer 1: 1 H NMR (400MHz, CDCl 3 )δ6.15(s,1H),5.42(s,1H),4.90(dd,J=12.2,6.8Hz,1H),4.84(s,1H),4.33(dd,J=11.9,3.9Hz,1H ),3.07(br s,1H),2.65(dd,J=13.4,6.8Hz,1H),2.09(dd,J=13.4,9.3Hz,1H),1.98–1.86(m,2H),1.83–1.66 (m,2H),1.60–1.38(m,4H),1.27(s,3H),1.16(s,3H),1.12(s,3H); 13 C NMR (100MHz, C...
Embodiment 2
[0074] Embodiment 2: the preparation of the thiazole derivative of cyanine A
[0075] The reaction formula is as follows:
[0076]
Embodiment 21
[0077] Example 2.1: Preparation of 2-aminothiazole derivative II a of cerulein A
[0078] Weigh 82mg (0.2mmol) of the obtained 2-bromocyanin and 15mg (0.2mmol) of thiourea prepared in Example 1 and dissolve them in 2mL of ethanol, heat and reflux for 5h and then cool naturally, add 10mL of saturated NaHCO 3 Add 18 mL of distilled water after the solution was alkalized, extract 3 times with dichloromethane, 30 mL each time, combine the extracts, wash 2 times with saturated brine, dry with anhydrous sodium sulfate, filter, concentrate under reduced pressure, and perform silica gel column chromatography Separation and purification, eluting with petroleum ether / ethyl acetate 2 / 1, yielded 28.6 mg of a white solid, which is the 2-aminothiazole derivative II a of cyanine A in this example, with a yield of 36.8%. mp.254-256℃.
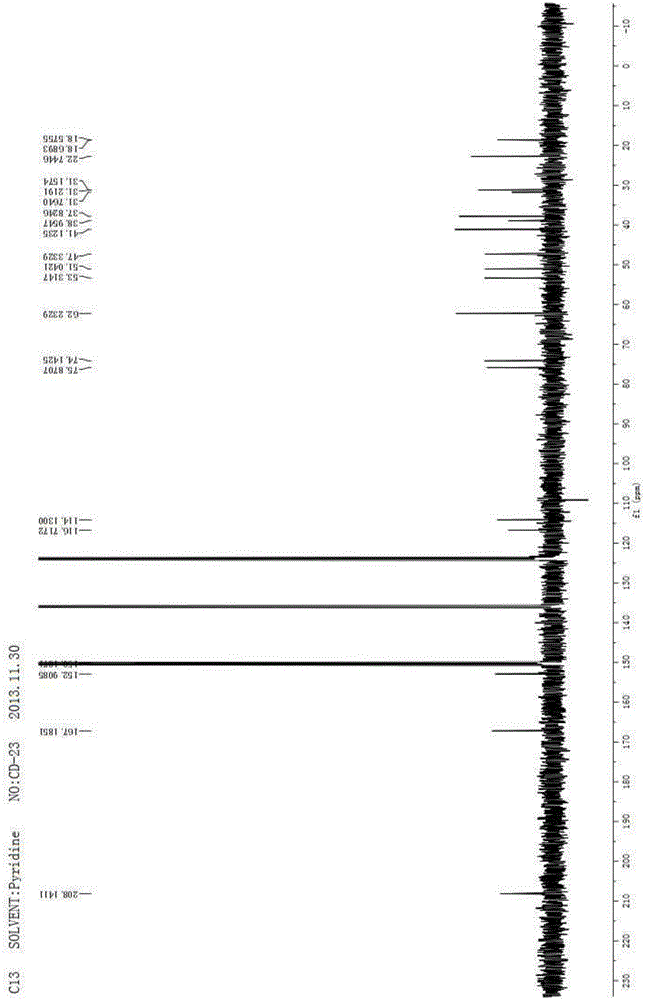
[0079] 1 H NMR (400MHz, C 5 D. 5 N)δ7.59(s,1H),7.26(s,1H),6.36(s,1H),5.42(s,1H),5.15(s,1H),4.90(s,1H),4.82(dt, J=11.9,4.0Hz,1H),3.29(br s,1H),2.61(d,J=15....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com