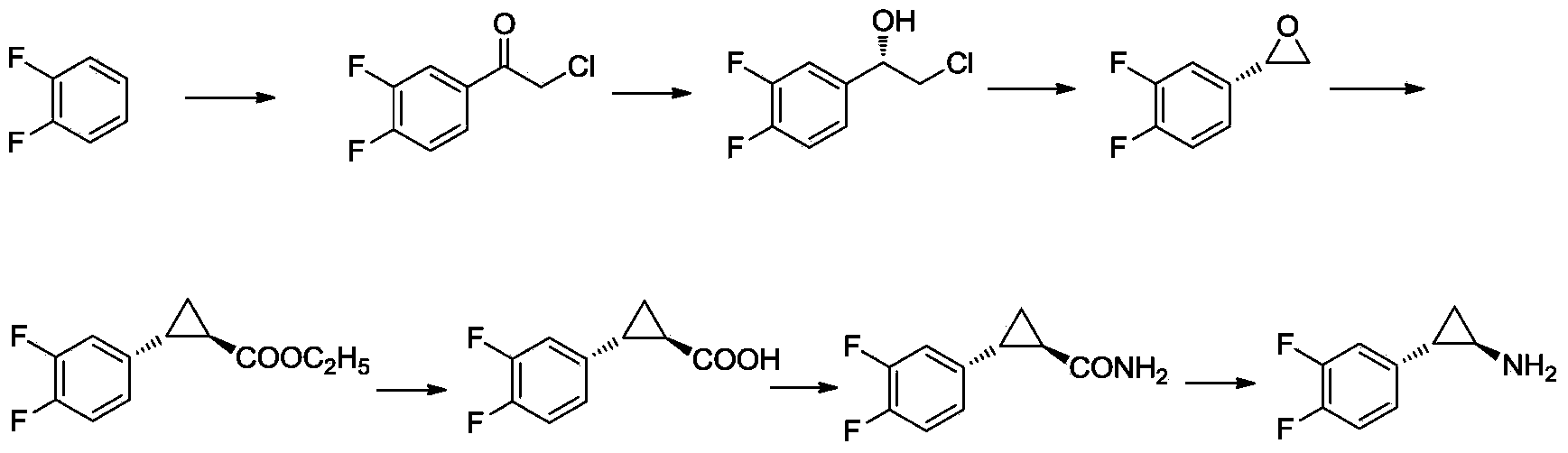
Method for preparing (1R,2S)-2-(3,4-difluorophenyl) -cyclopropylamine
A technology of difluorophenyl and cyclopropylamine, which is applied in the field of compound preparation, can solve the problems of low reduction stereoselectivity, low cyclopropanation yield, and high environmental protection pressure, and achieves low price, simple steps, and low environmental protection pressure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of 2-chloro-1-(2-bromo-4,5-difluorophenyl)ethanone (compound 3)
[0065]
[0066]Add 3,4-difluorobromobenzene (300g, 1.554mol, compound 2) into a 1L three-necked flask, protect it with argon, add aluminum trichloride (414.5g, 3.109mol), stir mechanically, and heat the oil bath to At 40°C, add chloroacetyl chloride (263.3g, 2.331mol) dropwise. After the drop is complete, stir mechanically at 40°C for 72h. HPLC (reverse-phase high-performance liquid chromatography) monitors the completion of the reaction. Slowly pour the reaction solution into ice water (2L ), stirred, added dichloromethane (1.8L) for extraction, and the organic phase was washed with saturated sodium bicarbonate (1.8L), water (1.8L*2), saturated brine (1.8L), and anhydrous sulfuric acid Sodium-dried and concentrated to obtain 418.6g of oil, which was distilled under reduced pressure (200Pa, external temperature 150°C) to obtain 339.3g of colorless oil (compound 3), yield: 81.0%, HPLC: Purit...
Embodiment 2
[0070] Preparation of 2-chloro-1-S-(2-bromo-4,5-difluorophenyl)ethanol (compound 4)
[0071]
[0072] Dissolve S-diphenylprolinol (4.18g, 0.0165mol) in toluene (180mL), add trimethyl borate (2.63mL, 0.023mol), stir at 20-30°C for 90min, add boron dropwise at 40°C Alkane dimethyl sulfide (20.1g, 0.264mol), temperature control 35 ~ 45 ℃, dropwise, stirred at 40 ℃ for 60min, then dropwise added 2-chloro-1-(2-bromo-4,5-difluorobenzene Base) ethyl ketone (89g, 0.33mol, compound 3 obtained in Example 1) in toluene (225mL) solution, temperature control 35 ~ 45 ° C, dropwise, stirred at 40 ° C for 60 min. TCL (thin-layer chromatography) monitoring (ethyl acetate:petroleum ether=1:20), after the reaction is complete, cool in an ice bath to 0-5°C, add methanol (100mL) dropwise, gas is released, stir for 30min, and spin down at 40°C Remove methanol, then spin off toluene with an oil pump to obtain 99g of oil, add dichloromethane (300mL) to dissolve, wash with 10% acetic acid aqueous ...
Embodiment 3
[0078] Preparation of (2S)-2-(2-bromo-4,5-difluorophenyl)oxirane (compound 5)
[0079]
[0080] Dissolve 2-chloro-1-S-(2-bromo-4,5-difluorophenyl)ethanol (96g, 0.354mol, compound 4 obtained in Example 2) in toluene (300mL), add hydrogen Sodium (21.2g, 0.53mol) in water (200mL) solution, stirred at 40°C for 1h, monitored by TCL (ethyl acetate:petroleum ether=1:10), after the reaction was completed, separated, leaving the toluene layer, water (200mL* 3) Wash and spin off toluene to obtain 74.4g of crude product, add n-heptane to dissolve it, quickly flush the column (1 times the amount of silica gel), dry over anhydrous sodium sulfate, and spin off n-heptane to get 67.2g of white solid (ie Compound 5), yield: 86.6% (calculated based on compound 3), HPLC: Purity=94.1%.
[0081] Compound 5 1 H NMR (CDCl 3 ,400MHz) δ(ppm): 7.38(m,1H);7.07(m,1H);4.06(t,1H);3.18(m,1H);2.60(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com