Method for directly synthesizing mesoporous material coated heteropolyacid functionalized MOF material
A technology of mesoporous materials and heteropolyacids, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, production of bulk chemicals, etc., to achieve high reaction yields, mild reaction conditions, and synthetic methods easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
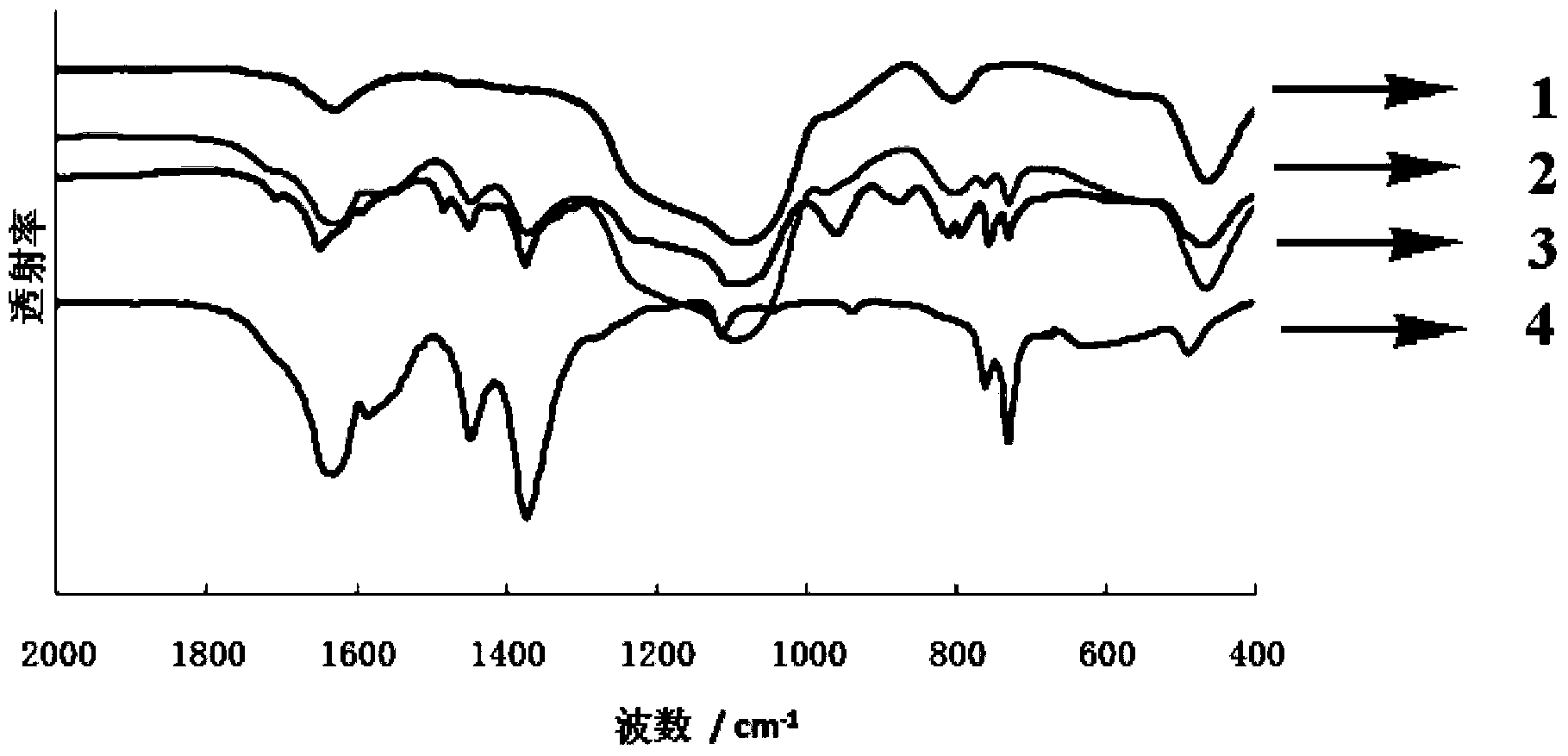
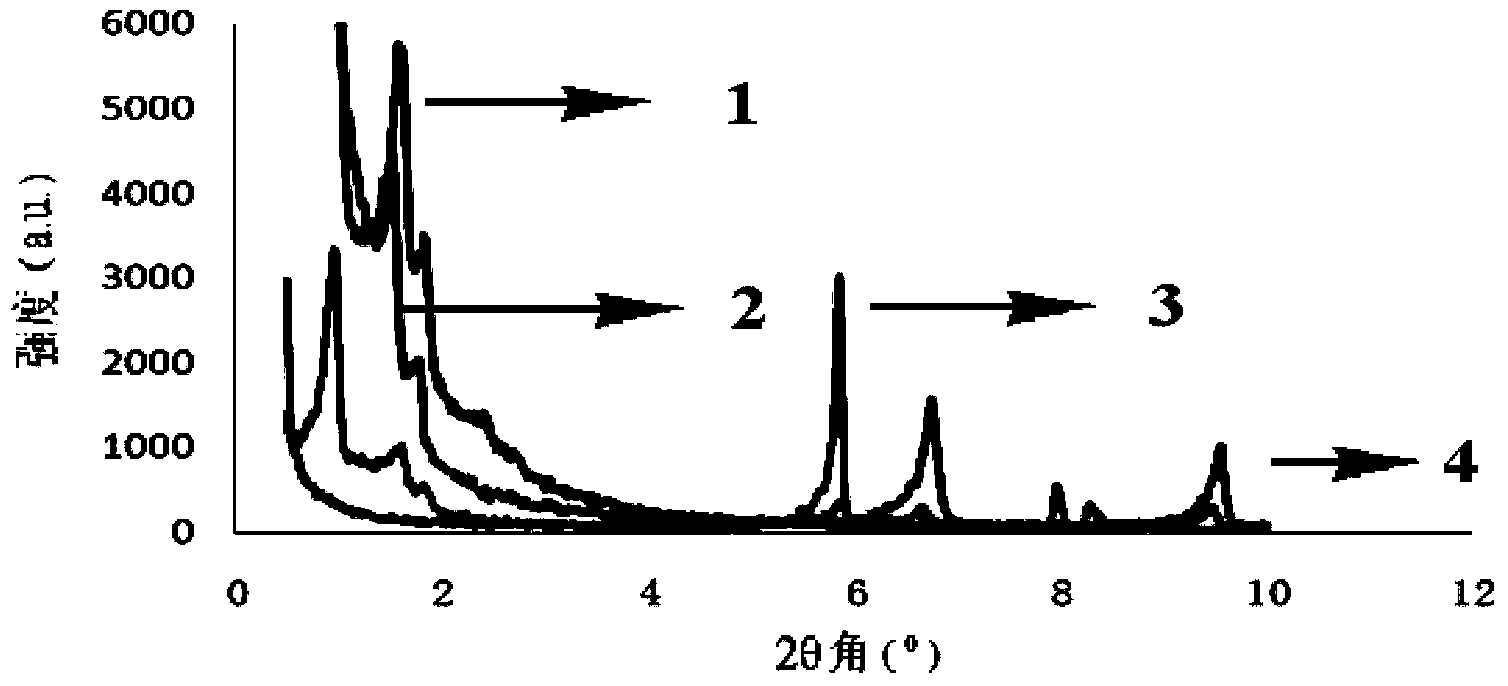
[0020] 0.180g SBA-15, 0.1062g H 4 PMo 11 VO 40 19H 2 O(0.05mmol), 0.240gCu(NO 3 ) 2 ·3H 2 O (1 mmol), 0.105 g of 1,3,5-trimesic acid (0.5 mmol), 0.09 g of tetramethylammonium hydroxide (1 mmol) were dissolved in 10 mL of deionized water, and the pH was adjusted to 2.5 with 2M NaOH. The solution was transferred to a 23mL autoclave, the temperature was programmed to rise at 5°C / h, and then raised to 160°C for 48h. After slowly cooling down to room temperature, the target material was precipitated from the reaction solution in the form of crystals. The obtained material was washed with water, and its structure was determined by XRD single crystal diffraction (the specific structure is as follows: figure 1 Shown), the mass yield of the heteropolyacid material with dual active centers is 35% by calculating the mass.
[0021] Using the obtained catalytic material for benzene hydroxylation reaction, 0.025mmol catalyst, 0.78g benzene (10mmol), 6.8mL acetonitrile, 0.9g ascorbic ...
Embodiment 2
[0023] 0.20g SBA-15, 0.2123g H 4 PMo 11 VO 40 19H 2 O(0.1mmol), 0.120gCu(NO 3 ) 2 ·3H 2 O (0.5 mmol), 0.105 g of 1,3,5-trimesic acid (0.5 mmol), 0.045 g of tetramethylammonium hydroxide (0.5 mmol) were dissolved in 10 mL of deionized water, and the pH was adjusted to 7.8 using 2M NaOH. The solution was transferred to a 23mL autoclave, the temperature was programmed to rise at 50°C / h, and then raised to 120°C for 72h. After slowly cooling down to room temperature, the target material was precipitated from the reaction solution in the form of crystals. The obtained material was washed with water, and its structure was determined by XRD single crystal diffraction (the specific structure is as follows: figure 1 shown), the mass yield of the heteropolyacid material with dual active centers is 25% by calculating the mass.
[0024] Using the obtained catalytic material for benzene hydroxylation reaction, 0.10g catalyst, 0.78g benzene (10mmol), 6.8mL acetonitrile, 0.9g ascorbic...
Embodiment 3
[0026] 0.16g SBA-15, 0.2123g H 4 PMo 11 VO 40 19H 2 O(0.1mmol),0.240gCu(NO 3 ) 2 ·3H 2 O (1 mmol), 0.105 g of 1,3,5-trimesic acid (0.5 mmol), 0.18 g of tetramethylammonium hydroxide (2 mmol) were dissolved in 10 mL of deionized water, and the pH was adjusted to 4.8 using 2M NaOH. The solution was transferred to a 46mL autoclave, the temperature was programmed to rise at 20°C / h, and then raised to 240°C for 16h. After slowly cooling down to room temperature, the target material was precipitated from the reaction solution in the form of crystals. The obtained material was washed with water, and its structure was determined by XRD single crystal diffraction (the specific structure is as follows: figure 1 Shown), the mass yield of the heteropolyacid material with dual active centers is 42% by calculating the mass.
[0027] Using the obtained catalytic material for benzene hydroxylation reaction, 0.20g catalyst, 0.78g benzene (10mmol), 6.8mL acetonitrile, 0.9g ascorbic acid, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com