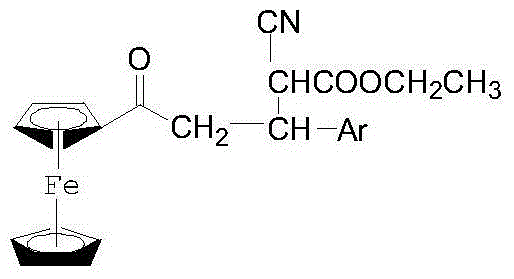
1-Ferrocenyl-aryl-3-(1-cyan-1-ethyl formate-methylene)-acetone and preparation method thereof
An ethyl formate-based, ferrocene-based technology, applied in chemical instruments and methods, metallocenes, antipyretics, etc., can solve the problems of low yield, long reaction time, large amount of solvent, etc. Simple process, short response time and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
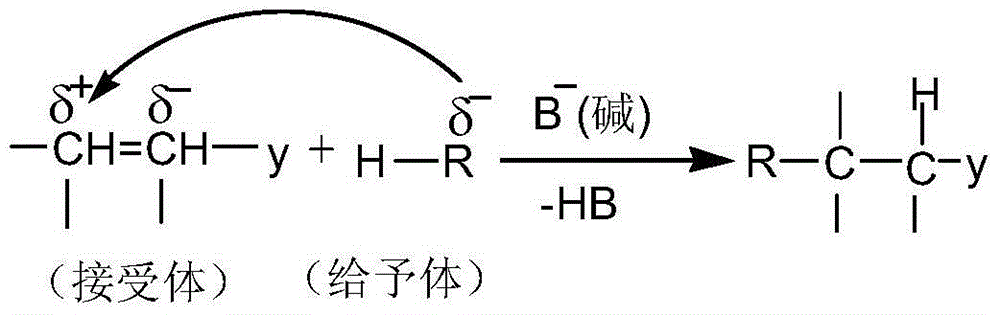
[0047] A preparation method of 1-ferrocenyl-3-aryl-3-(1-cyano-1-formyl ethyl-methine)-acetone, comprising the following steps:
[0048] Step 1) Amol 1-ferrocenyl-3-aryl-propenone, B mol anhydrous K 2 CO 3(or NaOH), C mol ethyl cyanoacetate was added into a dry mortar and ground rapidly at room temperature until the reaction was completed. The grinding time was 5-10 min. During the reaction, TLC was used to detect the reaction process. When 1-ferrocenyl-3-aryl When the raw material point of base-propenone disappears, it means that the raw material has completely reacted; the developer of TLC is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3. After the reaction is complete, the crude product is obtained.
[0049] Step 2) The crude product is washed with water and suction filtered several times. During the suction filtration, it is washed with water until the pH value of the filtrate is neutral, and the filter cake is vacuum-dried. The vacuum dry...
Embodiment 1
[0053] Weigh 0.0012mol ethyl cyanoacetate, 0.0012mol anhydrous K 2 CO 3 Put it in a mortar and mix quickly and evenly, then add 0.001mol 1-ferrocenyl-3-phenylpropenone, mix and grind at room temperature for 5 minutes. The mixture will start to become viscous as the reaction progresses, continue to grind until the material no longer changes, monitor the progress of the reaction using thin layer chromatography (TLC), when the starting point of 1-ferrocenyl-3-phenylpropenone disappears Indicates that the raw materials have completely reacted. After the reaction is completed, add a small amount of ethyl acetate to dissolve, filter, and concentrate the filtrate to dryness to obtain a dark red solid, which is 1-ferrocenyl-3-phenyl-3-(1-cyano- 1-Ethylcarboxy-methine)-acetone. m.p. (melting point): 62°C-63°C.
[0054] IR (KBr tablet) ν (cm-1): 2983, 2260, 1783, 1665, 1370, 1345; 1 H NMR: 7.29-7.68 (m, 5H, Ar-H), 4.39-4.94 (m, 9H, Cp), 3.63-3.96 (d, 1H, CH), 3.32-3.40 (m, 1H, CH), ...
Embodiment 2
[0056] Weigh 0.0012mol ethyl cyanoacetate, 0.0012mol anhydrous K 2 CO 3 Place in a mortar and mix quickly and evenly, then add 0.001mol 1-ferrocenyl-3-(p-chlorophenyl)-propenone, mix and grind at room temperature for 10 minutes. The mixture will start to become viscous as the reaction progresses, continue to grind until the substance no longer changes, use thin layer chromatography to monitor the reaction progress, when the raw material point of 1-ferrocenyl-3-(p-chlorophenyl)-propenone When it disappears, it means that the raw material has completely reacted. After the reaction is completed, add a small amount of ethyl acetate to dissolve, filter, and concentrate the filtrate to dryness to obtain a dark red solid, which is 1-ferrocenyl-3-(p-chlorophenyl)-3- (1-Cyano-1-carboxyethyl-methine)-acetone. m.p.: 75°C-76°C.
[0057] IR (KBr tablet) ν (cm -1 ): 3090, 2253, 1738, 1653, 1372, 1348, 820; 1 H NMR: 7.14-7.76 (m, 5H, Ar-H), 4.38-5.28 (m, 9H, Cp), 4.11-4.19 (d, 1H, CH), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com