Naphthalene derivative and application thereof in drugs
A drug and pharmaceutical technology, applied in the treatment of central nervous system dysfunction, in the field of naphthalene compounds of melatonin receptor agonists, can solve problems such as shortening sleep latency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
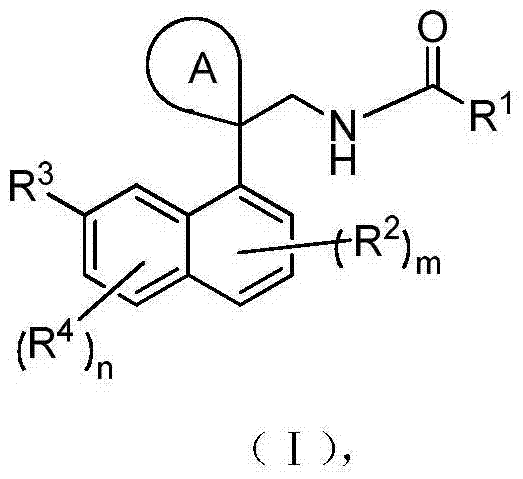
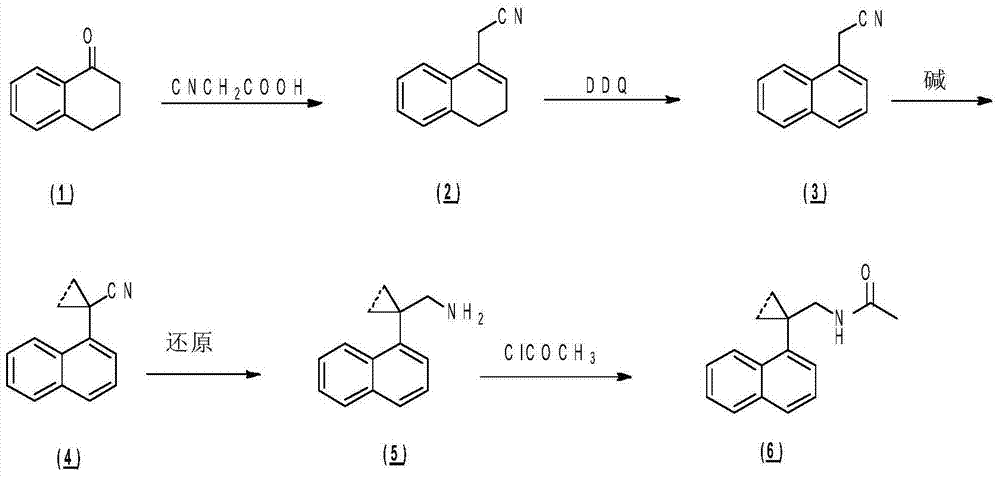
[0219] Embodiment 1N-((1-(naphthalene-1-yl) cyclopropyl) methyl) acetamide
[0220]
[0221] Step 1) Synthesis of 1-(naphthalene-1-yl)cyclopropanecarbonitrile
[0222] At -30°C, 2-(naphthalene-1-yl)acetonitrile (500mg, 2.99mmol) and sodium hydride (60%, 598mg, 14.95mmol) were added to 10mL of anhydrous DMF, and after stirring for 1h, slowly drop Add 1,2-dibromoethane (0.52 mL, 5.98 mmol, dissolved in 10 mL DMF), slowly raise the temperature to 25°C, and react for 1 hour. The reaction was stopped, quenched by adding 30 mL of saturated brine, extracted with ethyl acetate (30 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the filtrate was spin-dried under reduced pressure and purified by column chromatography (petroleum ether / ethyl acetate (v / v)=50 / 1) to obtain the title compound as a white solid (325 mg, 56.2%).
[0223] MS(ESI,pos.ion)m / z:194.2[M+1] + ;
[0224] 1 H NMR (400MHz, CDCl 3 )δ8.41(d, J=8.4Hz, 1H), 7....
Embodiment 2
[0234] Embodiment 2N-((1-(7-methoxynaphthalene-1-yl)cyclopropyl)methyl)acetamide
[0235]
[0236] Step 1) Synthesis of 1-(7-methoxynaphthalene-1-yl)cyclopropanecarbonitrile
[0237] The title compound of this step was prepared by referring to the method described in Step 1 of Example 1, that is, 2-(7-methoxynaphthalene-1-yl)acetonitrile (1.0g, 5.07mmol), sodium hydride (60%, 1.01g, 25.35 mmol) and 1,2-dibromoethane (0.88mL, 10.14mmol, dissolved in 10mL DMF) were prepared by reacting in anhydrous DMF (10mL), and the crude product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=50 / 1), concentrated and dried to obtain the title compound as a white solid (615 mg, 54.4%).
[0238] MS(ESI,pos.ion)m / z:224.1[M+1] + ;
[0239] 1 H NMR (400MHz, CDCl 3 )δ7.79 (t, J = 9.2Hz, 2H), 7.64 (d, J = 2.0Hz, 1H), 7.46 (dd, J = 6.8, 0.8Hz, 1H), 7.28 (dd, J = 7.2, 8.0 Hz, 1H), 7.24 (dd, J = 8.8, 2.4Hz, 1H), 4.02 (s, 3H), 1.84 (q, J = 4.8Hz, 2H), ...
Embodiment 3
[0248] Embodiment 3N-((1-(7-fluoronaphthalene-1-yl)cyclopropyl)methyl)acetamide
[0249]
[0250] Step 1) Synthesis of 1-(7-fluoronaphthalen-1-yl)cyclopropanecarbonitrile
[0251] The title compound of this step was prepared by referring to the method described in Step 1 of Example 1, that is, 2-(7-fluoronaphthalen-1-yl)acetonitrile (700mg, 3.78mmol), sodium hydride (60%, 756mg, 18.90mmol) and 1,2-Dibromoethane (0.65mL, 7.56mmol, dissolved in 10mL DMF) was prepared by reaction in anhydrous DMF (10mL), and the crude product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate (v / v) =50 / 1), concentrated and dried to give the title compound as a white solid (520 mg, 65.1%).
[0252] MS(ESI,pos.ion)m / z:212.1[M+1] + ;
[0253] 1 H NMR (400MHz, CDCl 3 )δ7.98(dd, J=10.4,1.2Hz,1H),7.90(dd,J=8.8,5.6Hz,1H),7.85(d,J=8.4Hz,1H),7.53(d,J=7.2 Hz, 1H), 7.40(t, J=7.6Hz, 1H), 7.34(td, J=8.8, 2.0Hz, 1H), 1.87(q, J=4.8Hz, 2H), 1.43(q, J=4.8 Hz, 2H).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com