Vaccine composition, and preparation method and application thereof
A vaccine composition and a technology for respiratory syndrome, applied in the field of veterinary biological products, can solve the problems of increased stress response of pigs, increased risk of infection, failure of immunization of pigs, etc., so as to reduce the cost of epidemic prevention and the effect of immunity. Excellent, high potency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
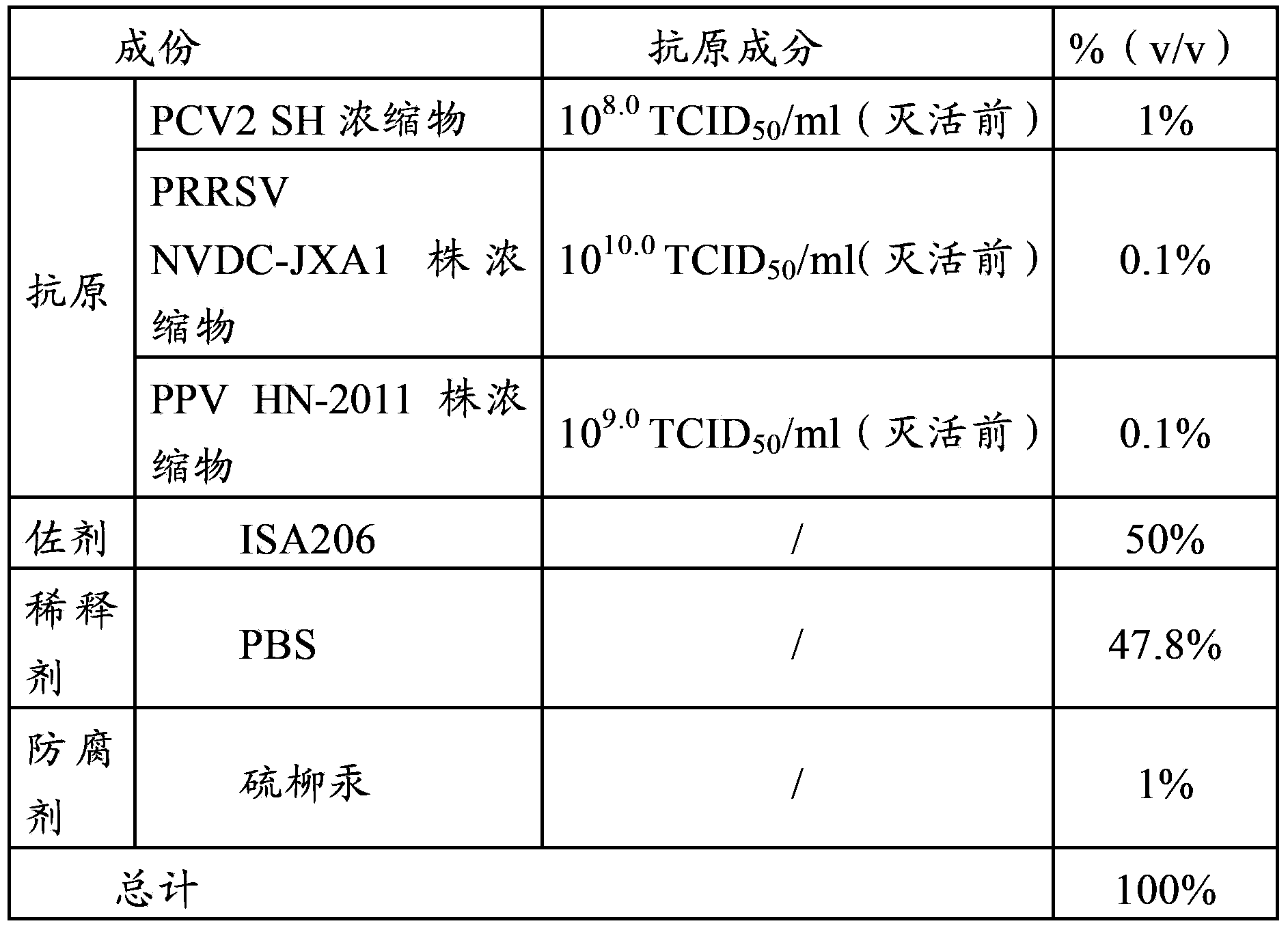
[0033] Example 1, Porcine Circovirus, Porcine Japanese Encephalitis Virus and Porcine Parvovirus Triple Inactivated Vaccine Composition, Preparation and Inspection of Porcine Japanese Encephalitis Virus and Porcine Parvovirus Dual Vaccine
[0034] 1. The source of the virus strain
[0035] The PCV2 virus described in the triple vaccine of the present invention is the PCV2SH strain, which has been preserved in the General Microbiology Center of the China Microbiological Culture Collection Management Committee. The preservation date is March 4, 2008, and the preservation number is CGMCC No2389.
[0036] The PRRSV strain described in the triple seedling of the present invention is the NVDC-JXA1 strain, which has been preserved in the General Microorganism Center of the China General Microorganism Culture Collection Management Committee. The preservation date: March 9, 2007, and the preservation number is CGMCC No1964.
[0037] The PPV strain described in the triple vaccine of the...
Embodiment 2
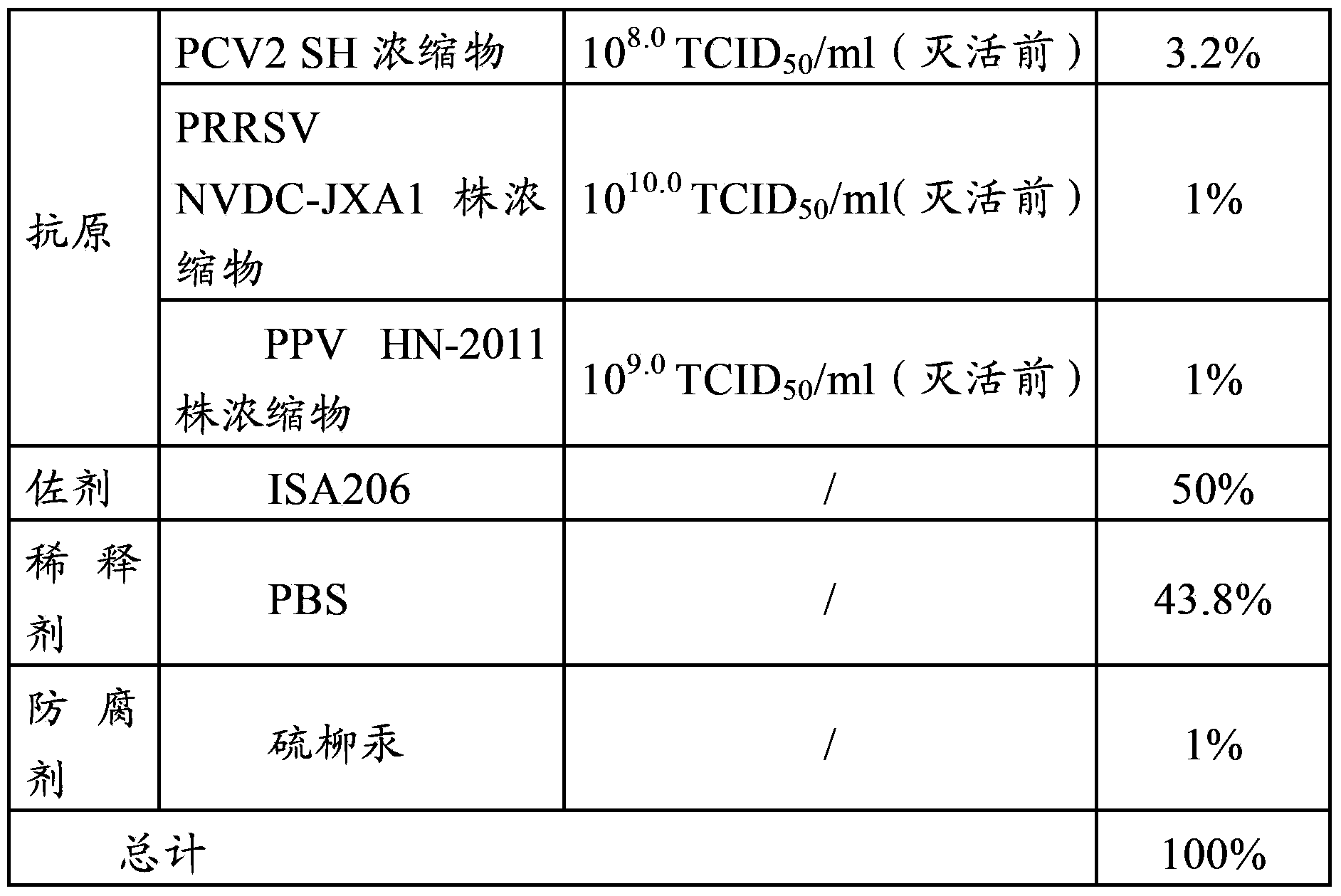
[0085] Embodiment 2, this study is to evaluate the immune efficacy of different antigen content PCV2-PRRSV-PPV combination vaccines and each virus single vaccine
[0086] 1. Test material
[0087] According to the method in embodiment 1, prepare PCV2-PRRSV-PPV triple vaccine (PCV2 antigen content is 10 6.0 TCID 50 / ml, PRRSV antigen content is 10 7.0 TCID 50 / ml, PPV antigen content is 10 6.0 TCID 50 / ml), recorded as vaccine L; PCV2-PRRSV-PPV triple vaccine (PCV2 antigen content is 10 6.5 TCID 50 / ml, PRRSV antigen content is 10 8.0 TCID 50 / ml, PPV antigen content is 10 7.0 TCID 50 / ml), recorded as vaccine M; PCV2-PRRSV-PPV triple vaccine (PCV2 antigen content is 10 7.0 TCID 50 / ml, PRRSV antigen content is 10 9.0 TCID 50 / ml, PPV antigen content is 10 8.0 TCID 50 / ml), recorded as vaccine H; low-dose PCV2-PRRSV-PPV triple vaccine (PCV2 antigen content is 5×10 5.5 TCID 50 / ml, PRRSV antigen content is 10 8.0 TCID 50 / ml, PPV antigen content is 10 7.0 TC...
Embodiment 3
[0115] Example 3, this study is to reproduce the sow reproductive disorder model under laboratory conditions
[0116] 1 Animal experiment design
[0117]Select 28 gilts and divide them into 7 groups with 4 gilts in each group. All sows were inoculated with 1ml each of PCV SH strain strong virus strain, PRRSV NVDC-JXA1 strong virus strain strain and PPV HN-2011 strong virus strain by intramuscular injection according to the experimental scheme described in Table 13, Observe the physiological state of the sows until farrowing. After delivery, observe whether there are symptoms of reproductive disorders such as miscarriage, stillbirth, mummified fetus, or fetal reabsorption. The presence or absence of PCV, PRRSV and PPV viruses in the internal organs of stillborn fetuses was detected; blood was collected from surviving fetuses to separate serum, and the presence of PCV, PRRSV and PPV antibodies was determined.
[0118] Table 13 Experimental scheme for replication of sow reprod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com