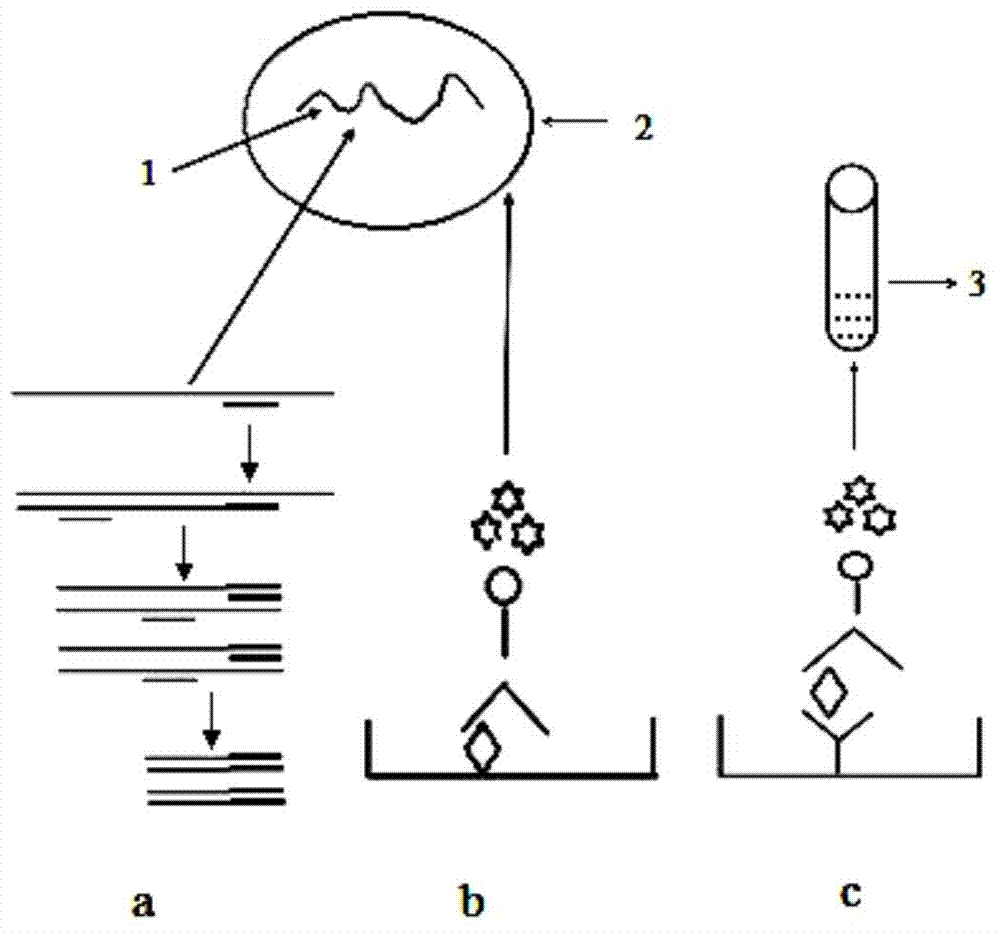
Composite murine hepatitis virus detection kit, detection method and application
A technology for detecting kits and hepatitis viruses, which is applied in the field of compound detection kits for rat hepatitis viruses, and can solve problems such as the inability to uniformly detect false positive rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] 3. Preparation of antigenic protein S2:
[0095] (1) Design the antigenic protein construction scheme:
[0096] The antigenic protein used in the present invention is that we obtained 6 main subtypes (MHV-1, MHV-2, MHV-3, JHM, A59 and S) of murine hepatitis virus prevalent at present by retrieval and analysis of the National Biotechnology Information Center of the United States Commonly unique to the stable domain S2 region of structural protein S (gi|298199707). The sequence of the S2 antigen protein is shown as SEQ ID NO.4 in the sequence listing, and the theoretical protein size is 50.4 kDa. The nucleic acid sequence corresponding to the S2 antigen protein sequence is shown in SEQ ID NO.5 in the sequence listing, and the gene size is 1383bp. Primers were designed according to the nucleic acid sequence of the antigenic protein S2. The upstream primer S2-f contained a NcoI restriction endonuclease site, and the sequence was shown in SEQ ID NO.6, 5′-CATGCCATGGCATGGATG...
Embodiment 1
[0115] Embodiment 1: Using the composite kit of the present invention to quickly and accurately detect murine hepatitis virus in mouse blood
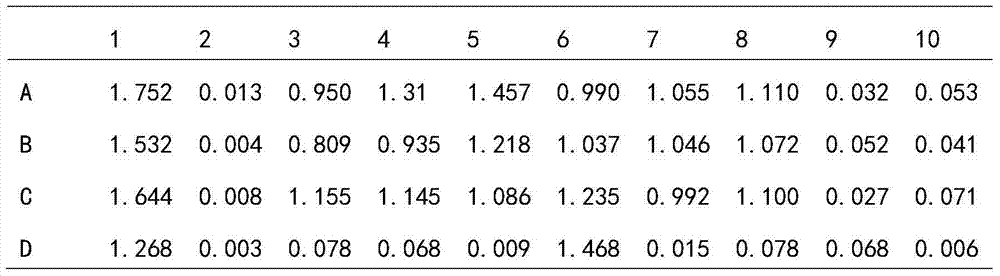
[0116] Using mouse blood as the sample to be tested, one-step RT-PCR, rapid indirect ELISA and rapid double-antibody sandwich ELISA were used to detect the presence of murine hepatitis virus and the antibodies produced by the virus in experimental mice Condition. Blood samples were collected from one chronically MHV-infected ICR mouse and two newly infected mice with normal immune function in experimental group 1, one chronically MHV-infected ICR mouse and two newly infected mice in experimental group 2 SCID mice and 2 negative virus-free mice in the control group.
[0117] (1) Sample processing: Collect 5-10 μl of blood from the tail or back vein of each mouse into 50 μl of PBS solution containing 5-10 units of heparin (anticoagulant) and 1 unit / ml DNase I.
[0118] (2) One-step RT-PCR detection of mouse blood samples:
[0119] Reag...
Embodiment 2
[0153] Embodiment 2: Using the composite kit of the present invention to quickly and accurately detect murine hepatitis virus in mouse saliva
[0154] Using mouse saliva as the test sample, one-step RT-PCR and rapid indirect ELISA were used to detect the presence of murine hepatitis virus. Saliva samples were collected from one chronically MHV-infected ICR mouse and two newly infected mice with normal immune function in experimental group 1, one chronically MHV-infected ICR mouse and two newly infected mice in experimental group 2, respectively. SCID mice and 2 negative virus-free mice in the control group.
[0155] (1) Sample treatment: 10 μl of saliva from the above-mentioned mice were collected respectively into 50 μl of PBS solution containing 1 unit / ml DNase I.
[0156] (2) One-step RT-PCR detection of mouse saliva samples:
[0157] Sample number: positive control (No. 11), negative control (No. 12), saliva of 1 chronic MHV-infected ICR mouse in experimental group 1 (No...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com