Traditional Chinese medicine effervescent tablet for treating respiratory diseases and preparation method of traditional Chinese medicine effervescent tablet
A technology for respiratory tract and effervescent tablets, which can be applied to respiratory diseases, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as inability to fully satisfy clinical medication, patient carrying, inconvenient taking, and large economic burden for patients. To achieve significant curative effect, fast drug dissolution and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
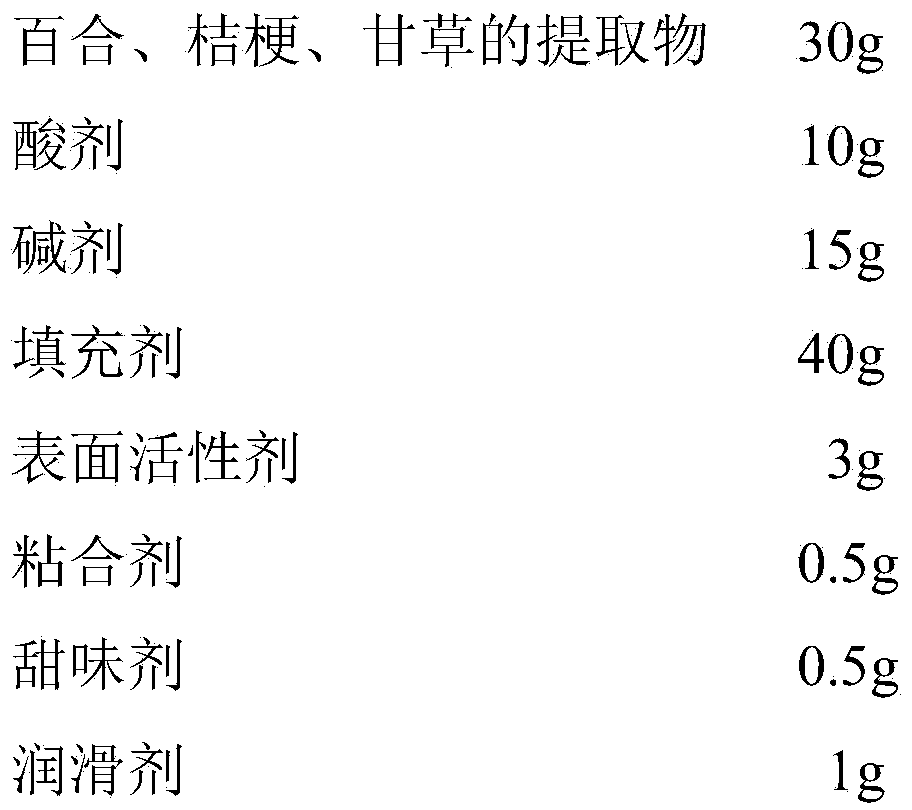
[0027] A traditional Chinese medicine effervescent tablet for treating respiratory diseases, which is composed of the following ingredients in weight proportions:
[0028]
[0029] The extracts of lily, platycodon, and licorice are water extracts of lily, platycodon, and licorice. , Platycodon grandiflorum, and licorice are boiled 3 times with water for 1.5 hours each time, combined with the decocting liquid, concentrated into extract, dried and pulverized.
[0030] The acid agent is tartaric acid.
[0031] The alkali agent is sodium bicarbonate.
[0032] The filler is lactose.
[0033] The surfactant was sodium lauryl sulfate.
[0034] The binder was povidone K30.
[0035] The sweetener is aspartame.
[0036] The lubricant was polyethylene glycol 6000.
[0037] The preparation method is as follows: the extracts of lily, platycodon, and licorice are dried and pulverized into fine powder with a particle size of 100 meshes (referring to the fine powder that can pass thr...
Embodiment 2
[0045] A traditional Chinese medicine effervescent tablet for treating respiratory diseases, which is composed of the following ingredients in weight proportions:
[0046]
[0047] The extracts of lily, platycodon, and licorice are water extracts of lily, platycodon, and licorice. , Platycodon grandiflorum, and licorice were decocted twice with water for 2 hours each time. Combine the decoction liquid and concentrate it into a clear paste with a relative density of 1.08. Add ethanol to the clear paste to make the alcohol content 50%. After stirring, let it stand for 24 minutes. hours, the supernatant was concentrated into extract, dried and pulverized.
[0048] The preparation method is as follows: the extracts of lily, Platycodon grandiflorum and licorice are dried and then pulverized into fine powder, and the dry powder of citric acid, 60% sodium bicarbonate, sucrose powder, sodium lauryl sulfate and stevioside is added, and the mixture is evenly mixed; After dissolving ...
Embodiment 3
[0053] A traditional Chinese medicine effervescent tablet for treating respiratory diseases, which is composed of the following ingredients in weight proportions:
[0054]
[0055] The extracts of lily, platycodon, and licorice are water extracts of lily, platycodon, and licorice, and the weight ratio of lily, platycodon, and licorice is 5:4:2 in turn, and extracted according to the conventional water decocting method.
[0056] The preparation method is as follows: the extracts of lily, platycodon and licorice are dried and then pulverized into fine powder, and the dry powders of tartaric acid, 80% sodium bicarbonate, lactose, sodium lauryl sulfate and protein sugar are added and mixed evenly; Bomb was dissolved in absolute ethanol and added to the above powder, mixed and granulated, added polyethylene glycol 6000 and the remaining 20% sodium carbonate to the granules, mixed and pressed into a ring-shaped tablet with a specification of 0.7g .
[0057] Tested: the disintegr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com