A kind of stable i crystal form clopidogrel bisulfate tablet and preparation method thereof
A technology of clopidogrel hydrogen sulfate tablet and clopidogrel hydrogen sulfate, applied in the field of medicine, can solve problems such as problems affecting drug effectiveness and safety, and achieve the effect of ensuring safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] 1. Pregelatinized starch, colloidal silicon dioxide, 971P passed through a 50-mesh sieve for later use;
[0046] 2. Dry the above-mentioned excipients that have been sieved at 100°C for 1 hour to remove moisture, and check that the moisture content is less than 3%.
[0047] 3. The I crystal form clopidogrel bisulfate, spray-dried lactose monohydrate, 90. Mix the pregelatinized starch evenly.
[0048] 4. Take the above-mentioned part of the material and colloidal silica through a 50-mesh sieve and mix evenly, and then add the sieved material back to the mixed material in the second step and mix evenly.
[0049] 5. Add the prescribed amount of sodium stearate fumarate and talcum powder, mix evenly again, and press into tablets.
[0050] 6. Remove the fine powder from the tablet core and carry out film coating to obtain the finished product.
Embodiment 2
[0052]
[0053] 1. Pass the prescribed amount of pregelatinized starch, colloidal silicon dioxide, and HPC-SL through an 80-mesh sieve for later use;
[0054] 2. Dry the above-mentioned excipients that have been sieved at 60°C for 4 hours to remove moisture, and check that the moisture content is less than 3%.
[0055] 3. The I crystal form clopidogrel bisulfate of prescription amount, 80. Mix the pregelatinized starch evenly.
[0056] 4. Take the above-mentioned part of the material and colloidal silica through a 60-mesh sieve and mix evenly, and then add the sieved material back to the mixed material in the second step and mix evenly.
[0057] 5. Add the prescribed amount of sodium stearate fumarate and talcum powder, mix evenly again, and press into tablets.
[0058] 6. Remove the fine powder from the tablet core and carry out film coating to obtain the finished product.
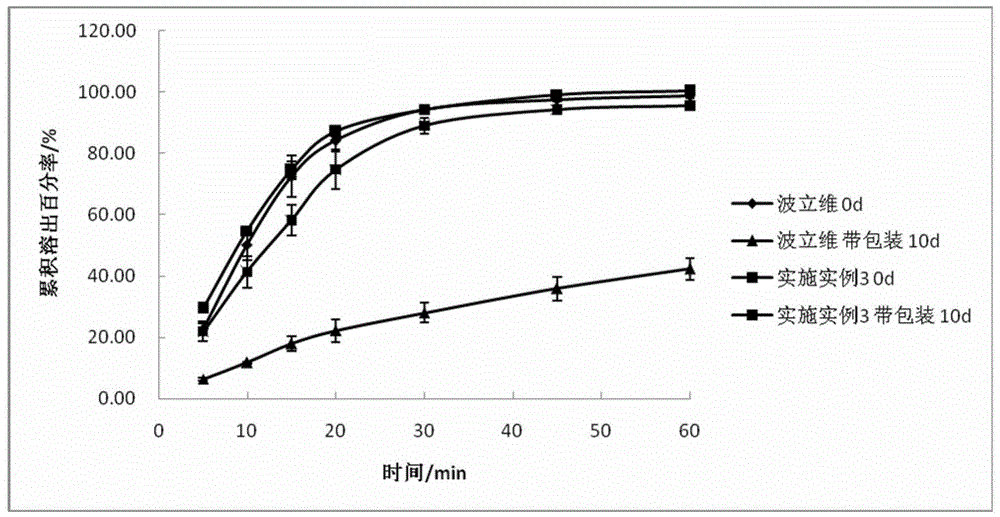
Embodiment 3
[0060]
[0061] 1. Pass the prescribed amount of pregelatinized starch, colloidal silicon dioxide, and HPC-SSL through a 60-mesh sieve for later use;
[0062] 2. Dry the above-mentioned excipients at 80°C for 2 hours to remove moisture, and check that the moisture content is less than 3%;
[0063] 3. Mix the prescribed amount of clopidogrel bisulfate in crystal form I, spray-dried lactose monohydrate, microcrystalline cellulose, pregelatinized starch, and HPC-SSL.
[0064] 4. Take the above-mentioned part of the material and colloidal silicon dioxide and pass through a 40-mesh sieve to mix evenly, and then add the sieved material back to the mixed material in the second step and mix evenly.
[0065] 5. Add the prescribed amount of sodium stearate fumarate and talcum powder, mix evenly again, and press into tablets.
[0066] 6. Remove the fine powder from the tablet core and carry out film coating to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com