Core-shell type LaFeO3@C lithium battery anode material and preparation method thereof
A negative electrode material and lithium battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as low cycle life, high irreversible capacity, and poor rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
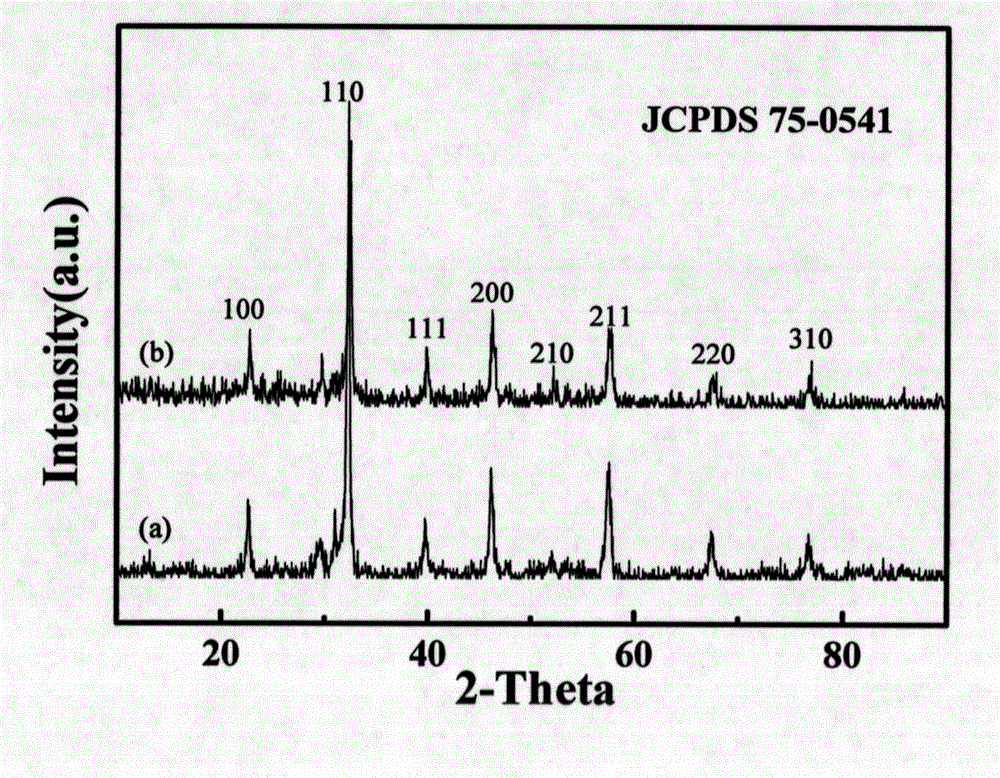
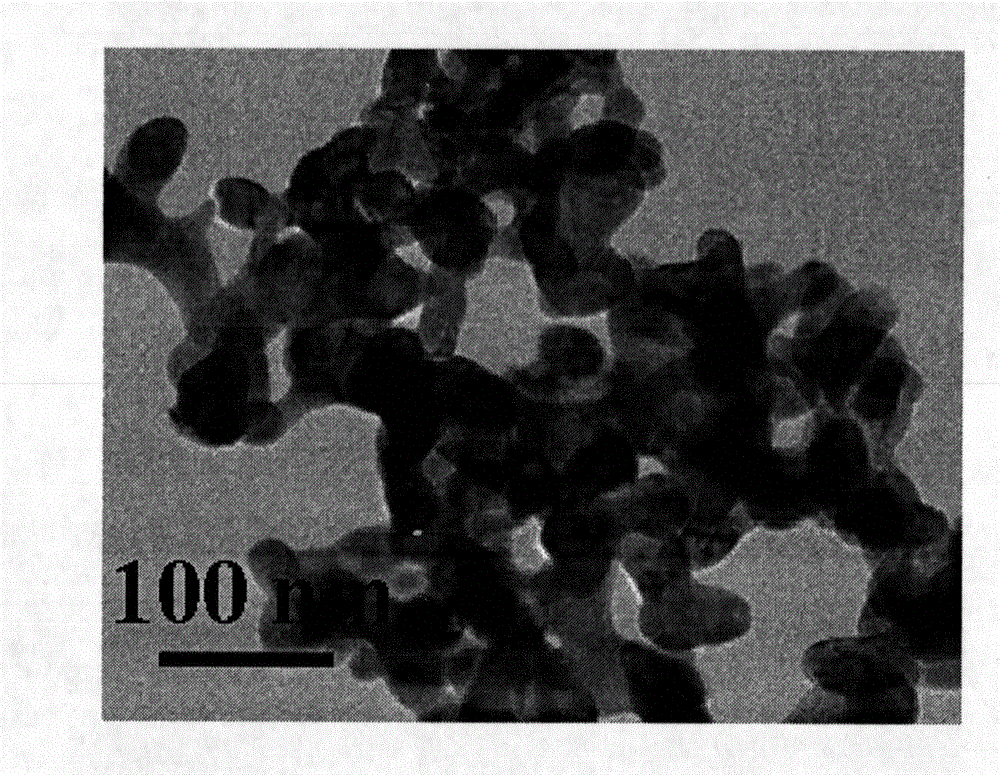
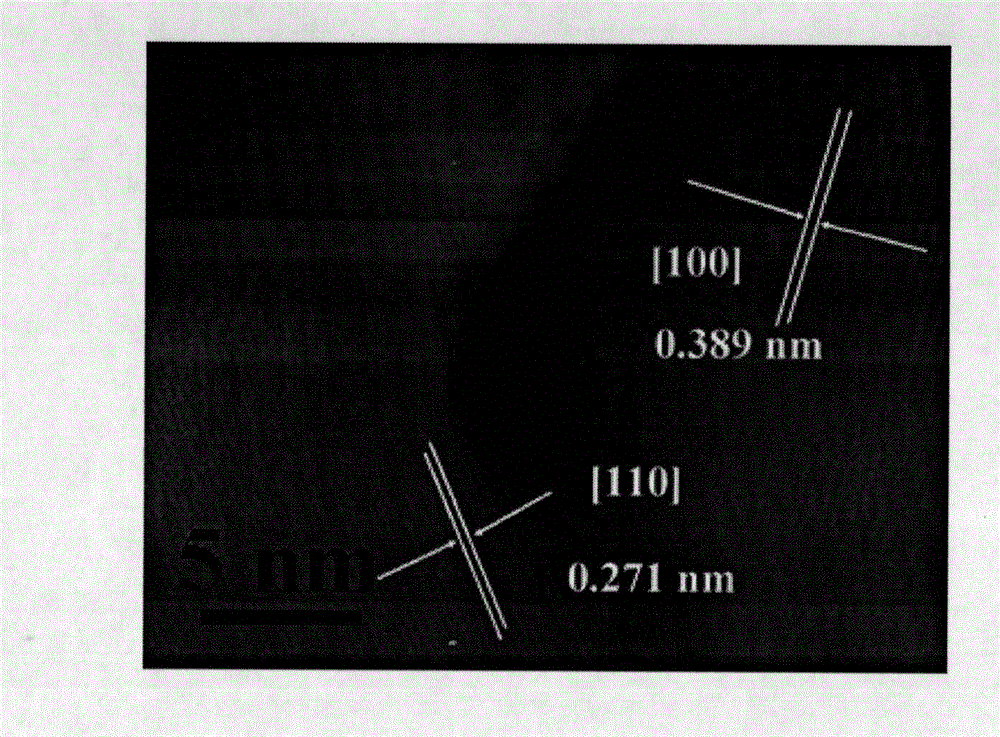
[0027] Embodiment 1: First weigh 0.22g La(NO 3 ) 3 ·6H 2 O, 0.20g Fe(NO 3 ) 3 9H 2 O, 0.3g urea, and 1.773g glucose monohydrate were dissolved in 35ml distilled water to obtain a pale yellow mixed solution. Transfer the above mixed solution to a hydrothermal reaction kettle, seal it well, raise the temperature to 180° C., keep it for 12 hours, and the reaction ends. After natural cooling, the solid product was collected, and the tan product received after washing and drying was the carbon-coated lanthanum iron precipitate nanomaterial. The above-mentioned tan product was calcined at 600°C for 4 hours under air and nitrogen atmosphere respectively, and the reddish-brown solid obtained by calcination under air was LaFeO 3, calcined under nitrogen to get a tan product which is LaFeO 3 C composite nanomaterials.
Embodiment 2
[0028] Embodiment 2: First weigh 0.22g La(NO 3 ) 3 ·6H 2 O, 0.20g Fe(NO 3 ) 3 9H 2 O, 0.45g urea, 1.773g glucose monohydrate were dissolved in 35ml distilled water to obtain a pale yellow mixed solution. Transfer the above mixed solution to a hydrothermal reaction kettle, seal it well, raise the temperature to 180° C., keep it for 12 hours, and the reaction ends. After natural cooling, the solid product was collected, and the tan product received after washing and drying was the carbon-coated lanthanum iron precipitate nanomaterial. The above-mentioned tan product was calcined at 800°C for 3 hours under air and nitrogen atmosphere respectively, and the reddish-brown solid obtained by calcination under air was LaFeO 3 , calcined under nitrogen to get a tan product which is LaFeO 3 C composite nanomaterials.
Embodiment 3
[0029] Embodiment 3: First weigh 0.22g La(NO 3 ) 3 ·6H 2 O, 0.20g Fe(NO 3 ) 3 9H 2 O, 0.6g urea, 1.773g glucose monohydrate were dissolved in 35ml distilled water to obtain a pale yellow mixed solution. Transfer the above mixed solution to a hydrothermal reaction kettle, seal it well, raise the temperature to 200° C., keep it for 12 hours, and the reaction ends. After natural cooling, the solid product was collected, and the tan product received after washing and drying was the carbon-coated lanthanum iron precipitate nanomaterial. The above-mentioned tan product was calcined at 1000°C for 2 hours under air and nitrogen atmosphere respectively, and the reddish-brown solid obtained by calcination under air was LaFeO 3 , calcined under nitrogen to get a tan product which is LaFeO 3 C composite nanomaterials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com