Making method of semi-quantitative tetrodotoxin detection card
A technology of tetrodotoxin and its production method, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of too specialized test strips, high cost of monoclonal antibodies, cumbersome instrument detection, etc. Small size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
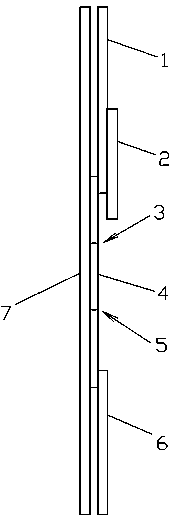
[0036] A semi-quantitative tetrodotoxin test card, such as figure 1 and figure 2 As shown in , it includes a carrier pad 7, a nitrocellulose membrane 4, a colloidal gold sample combination pad 1, an absorbent pad 6, an adhesive tape layer 2 and a cassette case.
[0037] The production process of the test card is divided into two steps, that is, the pretreatment of the early preparation and the later assembly. The pretreatment is mainly divided into the preparation of the colloidal gold sample combination pad and the processing of the chromatographic membrane. The specific steps are as follows:
[0038] 1) Preparation of colloidal gold sample combination pad 1: immunize mice with tetrodotoxin artificial antigen, take splenocytes from immunized mice and myeloma cells for fusion culture, screen positive hybridoma cells for in vitro culture, and clone them for expansion and culture and freeze them. BALB / c mice or their parental mice, choose BALB / c mice or their parental mice, fi...
Embodiment 2
[0045] The difference between embodiment 2 and embodiment 1 is:
[0046] 1) Preparation of colloidal gold sample combination pad 1: immunize mice with tetrodotoxin artificial antigen, take splenocytes from immunized mice and myeloma cells for fusion culture, screen positive hybridoma cells for in vitro culture, and clone them for expansion and culture and freeze them. BALB / c mice or their parental mice were selected, BALB / c mice or their parental mice were selected, and liquid paraffin was used for intraperitoneal injection of mice, and hybridoma cells were inoculated into the peritoneal cavity of mice 8 days later, and then 12 days later Collect the ascites, and then add 10 times the colloidal gold labeling solution to the ascites under the environment of pH11 for labeling. In order to avoid the formation of coagulated substances at the front end of the sample chromatography due to too many impurities in the ascites, prevent sample chromatography and combine with nitrocellulos...
Embodiment 3
[0050] The difference between embodiment 3 and embodiment 1 is:
[0051] 1) Preparation of colloidal gold sample combination mat: mice were immunized with tetrodotoxin artificial antigen, splenocytes from immunized mice were fused with myeloma cells for fusion culture, positive hybridoma cells were screened and cultured in vitro, clones were expanded and cultured and frozen, and BALB was selected / c mice or their parental mice, BALB / c mice or their parental mice were selected, and liquid paraffin was used for intraperitoneal injection of mice, and hybridoma cells were inoculated into the peritoneal cavity of mice 7 days later, and then collected 10 days later Ascites, and then add 15 times of colloidal gold labeling solution to the ascites under the environment of pH9 for labeling. In order to avoid the formation of coagulated substances at the front end of the sample chromatography due to too many impurities in the ascites, which prevents the sample from being chromatographed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Spacing | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com