Carboxylated fluorescent microsphere, preparing method thereof and applications of the carboxylated fluorescent microsphere
A fluorescent microsphere, carboxylation technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of rare reports of technical solutions, complicated preparation process of conjugated polymers, etc., to avoid post-functionalization reaction. , good monodispersity and size controllable, effective fixation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Preparation of CP1
[0036] References Journal of the American Chemical Society 2004 , 126, 14964; ACS Applied Materials & Interfaces The raw materials provided in 2014, 6, 5041, the polyarylene acetylene polymer CP1 with carboxyl groups in the side chain is prepared by Sonogashira coupling reaction, the specific method is as follows:
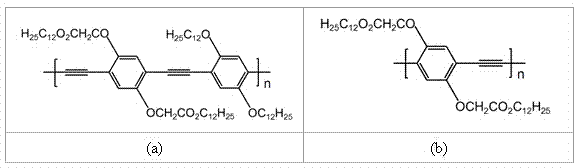
[0037] See attached figure 1 , wherein (a) is the structural formula of the raw material polymer Pre-CP1 of the present embodiment (in the literature ACS Applied Materials & Interfaces 2014, 6, 5041, Pre-CP1 is correspondingly denoted as P3), pre-CP1 (1.03 g) was dissolved in 1,4-dioxane (160 mL), and 1M n-Bu 4NOH methanol solution (10 mL), stirred at room temperature for 24 hours. During the hydrolysis of the polymer, a few drops of deionized water were added to keep the polymer in solution. After the reaction was completed, sodium perchlorate (1.22 g) was dissolved in 20 mL of water and added to the hydrolyzed polymer s...
Embodiment 2
[0047] References Journal of the American Chemical Society 2004 , 126, 14964; ACS Applied Materials & Interfaces The raw materials provided in 2014, 6, 5041, the polyarylene acetylene polymer CP2 with carboxyl groups in the side chain is prepared by Sonogashira coupling reaction, the specific method is as follows:
[0048] See attached figure 1 , where (b) is the structural formula of the raw material polymer Pre-CP2 of this embodiment (in the document ACS Appl. Mater. Interfaces 2014, 6, 5041, Pre-CP2 is correspondingly denoted as P4), and pre-CP2 (0.3 g) Dissolve in 1,4-dioxane (80 mL), add 1M n-Bu 4 NOH methanol solution (5 mL), stirred at room temperature for 24 hours. During the hydrolysis of the polymer, a few drops of deionized water were added to keep the polymer in solution. After the reaction was completed, sodium perchlorate (0.61 g) was dissolved in 10 mL of water and added to the hydrolyzed polymer solution, stirred, and the resulting mixture was poured ...
Embodiment 3
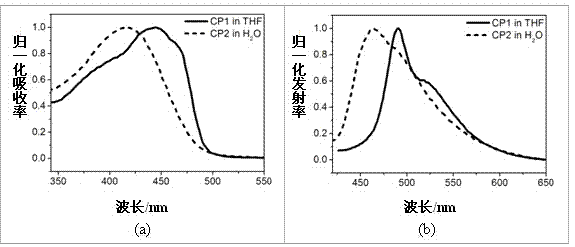
[0057] See attached Figure 5 , which is the solid absorption spectrum (left) and emission spectrum (right) of the fluorescent microspheres provided by the embodiment of the present invention with the reaction sites for bioconjugation.
[0058] The two kinds of fluorescent microspheres provided in Examples 1 and 2 of the present invention are tested for their ability to couple with biomolecules, and the specific method is as follows:
[0059] Preparation of APGMA-CP1 fluorescent microspheres loaded with FITC-BSA: Weigh a mixed solution of APGMA-CP1 fluorescent microspheres (0.01g) in phosphate buffer solution (5 mL, pH 5~6) and THF (3 mL) In , the carboxyl groups on the surface of the microspheres were activated (30 min) in the presence of EDC (0.045 g) and NHS (0.081 g). After the activation reaction was completed, adjust the pH to 8 using disodium hydrogen phosphate-sodium dihydrogen phosphate buffer, add BSA-FITC (0.05 g), and shake at 4°C in the dark (3 h*1000 r / min). Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com