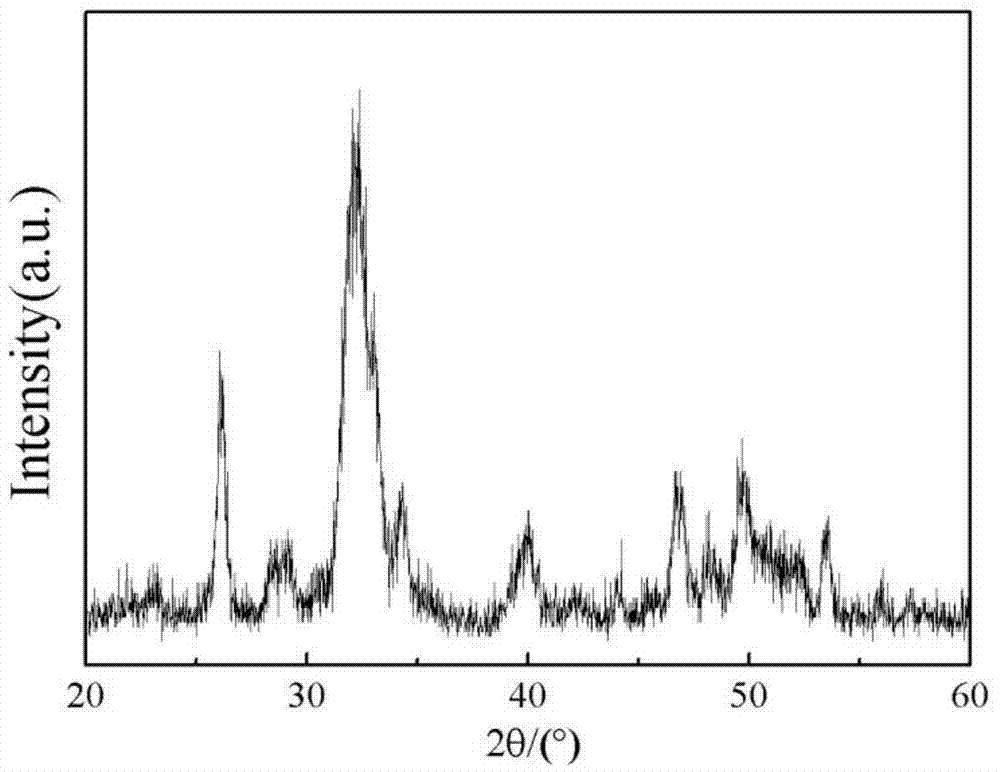
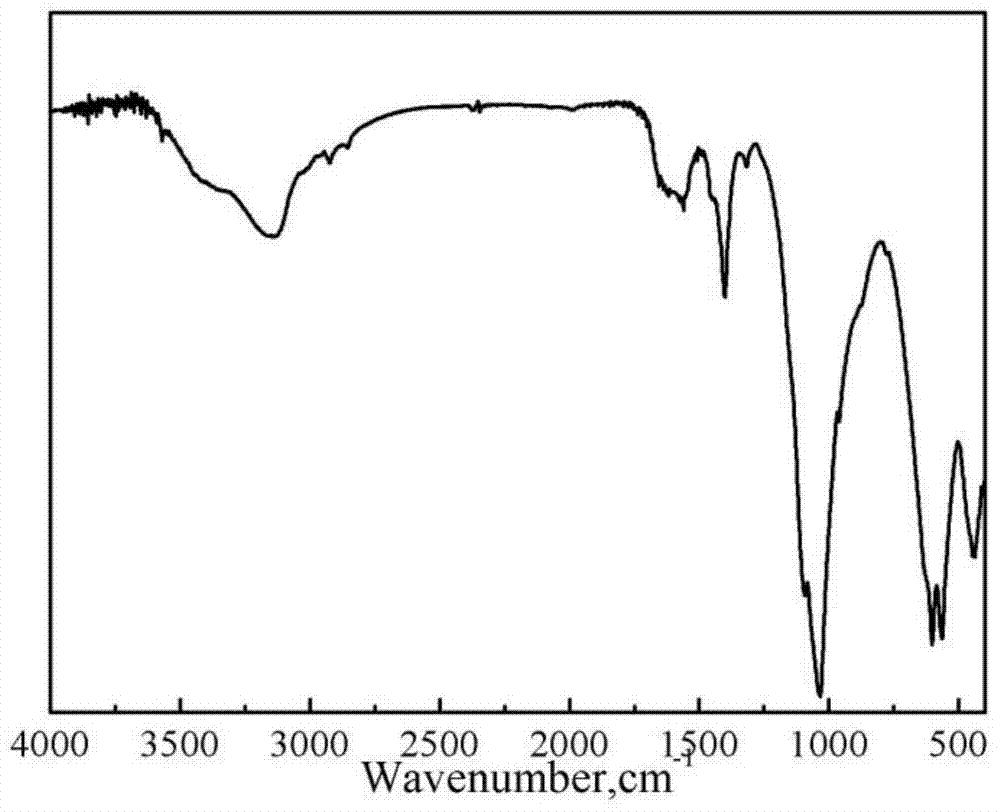
Magnetic hydroxyl apatite/polymer tridimensional porous stent material with oriented magnetic field and preparation method thereof
A hydroxyapatite, orientation magnetic field technology, applied in medical science, prosthesis, etc., can solve the problems of no orientation magnetic field, no macroporous structure, unsatisfactory osteoinductivity, etc., to promote the formation of new bone, The effect of improving osteoinductivity and improving osseointegration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of chitosan solution: 1 g of chitosan was dissolved in 25 mL of acetic acid solution (2 vol%), magnetically stirred for 12 h until completely dissolved, and ultrasonically removed for 1 h to remove air bubbles;
[0034] (2) Weigh 4.5g hydroxyapatite and 4.5g M-type ferrite (SrFe 12 o 19 ), placed in the chitosan solution, stirred at 450rpm for 20h until uniformly dispersed, and ultrasonically removed for 30min to remove air bubbles;
[0035] (3) Transfer the mixed solution containing chitosan, ferrite and hydroxyapatite to a 12mm×18mm (diameter×height) mold, freeze at -20°C for 10h under a uniform magnetic field of 1000Gs, and then transfer to freeze-drying freeze-dry at -80°C for 30 hours to obtain a magnetic hydroxyapatite / chitosan three-dimensional porous scaffold;
[0036] (4) At room temperature, soak the magnetic hydroxyapatite / chitosan three-dimensional porous scaffold in 100mL of 10wt% NaOH for 24 hours, wash it with deionized water for 6-8 tim...
Embodiment 2
[0040] The magnetic hydroxyapatite / chitosan three-dimensional porous scaffold obtained in Example 1 with an orientation magnetic field was tested for in vitro biological activity.
[0041] (1) Preparation of simulated body fluid: keep the temperature of deionized water in the plastic container at 37°C, add 8.035g NaCl and 0.355g NaHCO to the stirred deionized water in sequence 3 , 0.225g KCl, 0.231g K 2 HPO 4 ·3H 2 O, 0.311g MgCl 2 ·6H 2 O, 39mL1.0mol / LHCl, 0.292g CaCl 2 , 0.072g Na 2 SO 4 , then use 6.118g (CH 2 Oh) 3 CNH 2 , 1.0mol / L HCl to adjust the pH of the solution to 7.4, dilute to 1000mL, transfer to a plastic container, and store at 37°C for later use.
[0042] (2) According to the dosage ratio of 50mL / tablet, measure the simulated body fluid in a beaker, soak and transform at 37°C for 2 days, cool, wash and dry.
[0043] The morphology of the three-dimensional porous scaffold obtained in Example 2 was characterized, and the scanning electron microscope im...
Embodiment 3
[0045] CCK-8 method to study human bone marrow mesenchymal stem cells (hBMSCs) obtained in Example 1 with an orientation magnetic field magnetic hydroxyapatite / chitosan three-dimensional porous scaffold (Magnetic scallfolds)
[0046] And the growth of hydroxyapatite / chitosan three-dimensional porous scaffold (Normal scallfolds) material surface:
[0047] (1) Put each group of materials in a 24-well plate (the material is disc-shaped, with a diameter of 10 mm);
[0048] (2) Press 1×10 4 The density of cells / sample seeded hBMSCs on each group of materials;
[0049] (3) Add 100 μl of 5 μg / mL CCK-8 reaction solution at each time point (1d, 3d, 5d, 7d), and incubate in a constant temperature incubator at 37°C for 2 hours;
[0050] (4) Aspirate CCK-8 solution, 200 μl / well, add to 96-well plate. The OD value was measured with a microplate reader at a wavelength of 450 nm.
[0051] Figure 6 The CCK-8 method is used to evaluate the adhesion and value-added maps of human bone marr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com