Method for detecting Escherichia coli in water and detection culture solution
A technology of Escherichia coli and culture solution, which is applied in the direction of biochemical equipment and methods, and the determination/inspection of microorganisms, which can solve the problems affecting the detection accuracy and detection time, small sampling volume, undisclosed chromogenic medium, etc. problems, to achieve real-time monitoring of microbial contamination, reduced detection time, and short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: method of the present invention
[0038] Detection medium: Tryptone 10.0g, magnesium sulfate 50mg, manganese sulfate 0.5mg, zinc sulfate 0.5mg, ammonium sulfate 5.0g, sodium chloride 10.0g, calcium chloride 50mg, sodium sulfite 40mg, Tween-802g, NaH 2 PO 4 ·H 2 O6.10g, K 2 HPO 4 ·3H 2 O2.75g, o-nitrobenzene-β-D-galactopyranoside 250mg, 4-methylumbelliferone-β-D-glucuronide 35mg, No. 3 bile salt 3.0g.
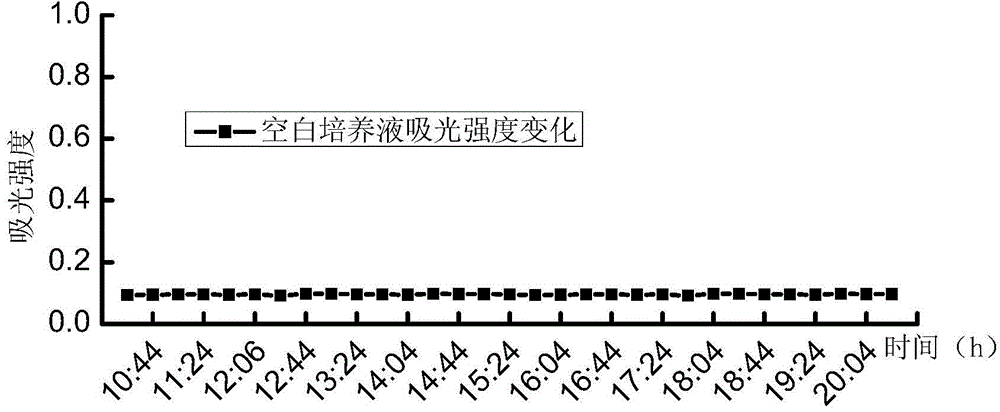
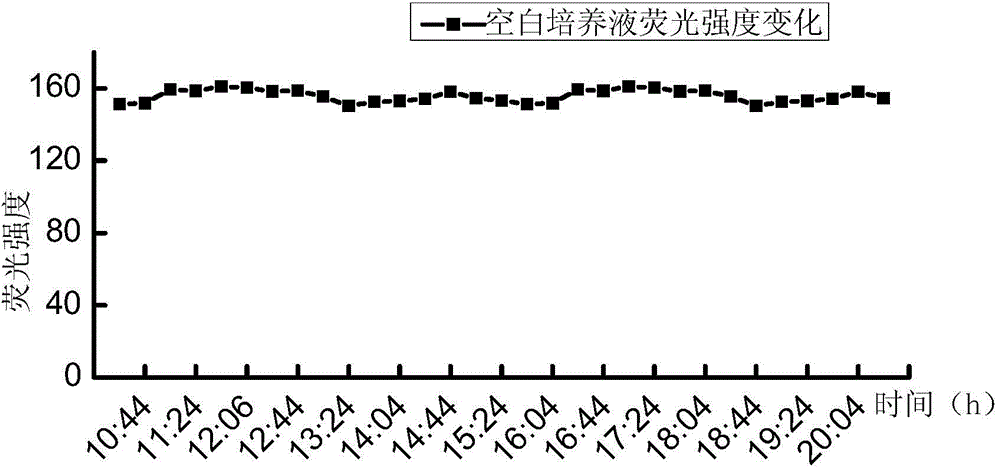
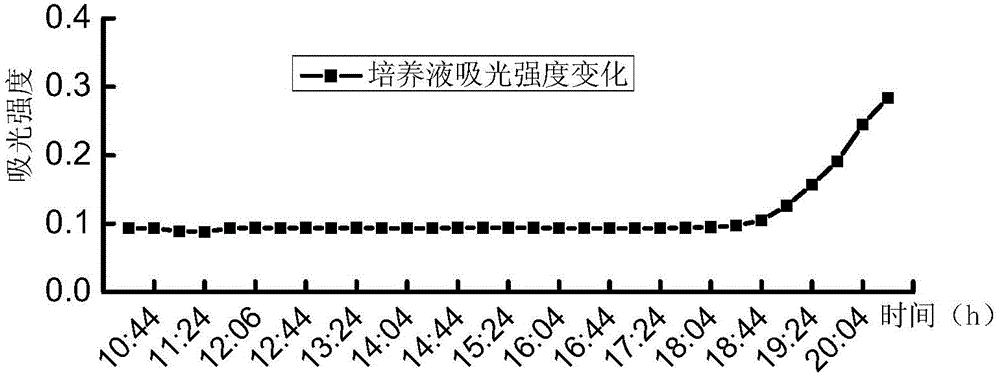
[0039] A series of suspensions of Escherichia coli with different concentrations (5.2, 52, 5.2×10 2 , 5.2×10 3 , 5.2×10 4 , 5.2×10 5 , 5.2×10 6 , 5.2×10 7 MPN / 100mL) each 100mL, first filter and concentrate through a cellulose acetate filter membrane with a membrane pore size of 0.45 μm and a membrane diameter of 47 mm, then add the filter membrane to the test culture solution for cultivation, the culture temperature is 37 ° C, and the culture solution is continuously tested during the culture period The 450nm fluorescence intensity produced under...
Embodiment 2
[0042] Embodiment 2: method of the present invention
[0043] Detection medium: Tryptone10.0g, magnesium sulfate 100mg, manganese sulfate 1mg, zinc sulfate 1mg, ammonium sulfate 10.0g, sodium chloride 10.0g, calcium chloride 100mg, sodium sulfite 100mg, Tween-800.5g, NaH 2 PO 4 ·H 2 O6.10g, K 2 HPO 4 ·3H 2 O2.75g, o-nitrobenzene-β-D-galactopyranoside 250mg, 4-methylumbelliferone-β-D-glucuronide 35mg, No. 3 bile salt 1.0g.
[0044] A series of suspensions of Escherichia coli with different concentrations (6.3, 63, 6.3×10 2 , 6.3×10 3 , 6.3×10 4 , 6.3×10 5 , 6.3×10 6 , 6.3×10 7 MPN / 100mL) each 100mL, first filter and concentrate through a cellulose acetate filter membrane with a membrane pore size of 0.45 μm and a membrane diameter of 47 mm, then add the filter membrane to the test culture solution for cultivation, the culture temperature is 37 ° C, and the culture solution is continuously tested during the culture period The 450nm fluorescence intensity produced unde...
Embodiment 3
[0047] Embodiment 3: comparison of detection time
[0048] Dilute with sterile water to prepare a series of Escherichia coli suspensions with different concentrations of 100mL each, first filter and concentrate through a cellulose acetate filter membrane with a membrane pore size of 0.45 μm and a membrane diameter of 47 mm, and add the filter membrane to the detection culture solution Cultivate at a temperature of 37°C. During the cultivation period, continuously detect the 450nm fluorescence intensity of the culture solution under the 365nm excitation light, and record the fluorescence intensity of the 450nm fluorescence produced by the Escherichia coli suspension at each concentration under the 365nm excitation light. 240 hours of time.
[0049] At the same time, according to the linear curve and linear equation of the patent 200910148688.7, the different concentrations of Escherichia coli suspensions prepared in this example were substituted into it, and the detection time ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com