Traditional Chinese medicine extract for treating osteoarthritis and preparation method thereof
A technology of osteoarthritis and extracts, applied in the field of traditional Chinese medicine extracts and preparations for the treatment of osteoarthritis, can solve problems such as unsatisfactory treatment effects, achieve significant social and economic benefits, broad application prospects, and significant curative effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of Chinese medicinal extracts for the treatment of osteoarthritis
[0025] (1) Law One
[0026] Take by weighing 25Kg of Sangjisi, 25Kg of Chuanduan, 15Kg of Qiannianjian, 15Kg of Pinus tabulaeformis, 15Kg of Drynaria fortunei, 10Kg of ground beetle, 15Kg of Achyranthes bidentata, and 25Kg of Spatholobus Spatholobus.
[0027] Mix the two medicines of Chinese pine knot and Qiannianjian, add water in an amount 8 times the total mass of the two medicines, add them into a multifunctional extraction tank, and extract for 5 hours to obtain the water extract 1 and the oil-water mixture. The oil-water mixture is separated by ultrafiltration using an organic membrane. The membrane material is a modified polysulfone membrane with a molecular weight cut-off of 120,000 Daltons, an operating pressure of 0.10 MPa, and a system temperature of 60°C. Take the ultrafiltration retentate (340mL) as the volatile oil, and the ultrafiltration permeate as the water extra...
Embodiment 2
[0036] Embodiment two clinical research
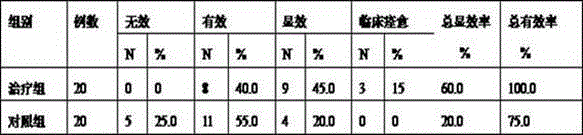
[0037] Select 40 patients with knee osteoarthritis, including 20 cases in the treatment group and 20 cases in the control group. Clinically observed the changes of laboratory indicators such as WOMAC index of main symptoms and signs, TCM symptoms and erythrocyte sedimentation rate in the two groups before and after treatment.
[0038] 1. Treatment method
[0039] (1) Treatment group: Gubi granule (prepared in Example 1 (1)), twice a day, 2 packs each time. If the joint pain is obvious, short-term application (3-5 days) of meloxicam tablets 7.5 mg can be taken as appropriate, 1 tablet each time, orally once a day.
[0040] (2) Control group: mainly used non-steroidal anti-inflammatory drugs, meloxicam tablets 7.5 mg, 1 tablet each time, orally once a day. If economic conditions permit, drugs to improve cartilage metabolism (glucosamine sulfate 0.25g-0.5g, orally 3 times a day) will be administered at the same time.
[0041] (3) Cour...
Embodiment 3
[0069] Example 3 Comparison of anti-inflammatory pharmacological experiments between the extract of the present invention and the extract obtained by other processes
[0070] 1 Materials and Instruments
[0071] 1.1 Experimental animals
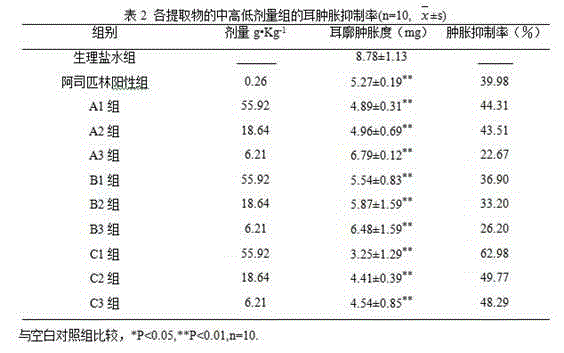
[0072] 220 adult SPF-grade ICR mice, half male and half female, weighing (25±2) g (Nanjing Qinglongshan Animal Breeding Farm, license number: SCXK (Shanghai) 2013-0012) half were used for the mouse ear swelling experiment, half Used in mouse toe swelling experiment. The mice were adaptively fed for 1 week, and the picric acid markers were divided into 11 groups: normal saline group, aspirin group, high-dose decoction group (A1), medium-dose decoction group (A2), and low-dose decoction group (A3), high-dose group of ethanol precipitation method (B1), medium-dose group of ethanol precipitation method (B2), low-dose group of ethanol precipitation method (B3), high-dose group of Gubi solution (C1), middle-dose group of Gubi solution Dose group...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Membrane pore size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com